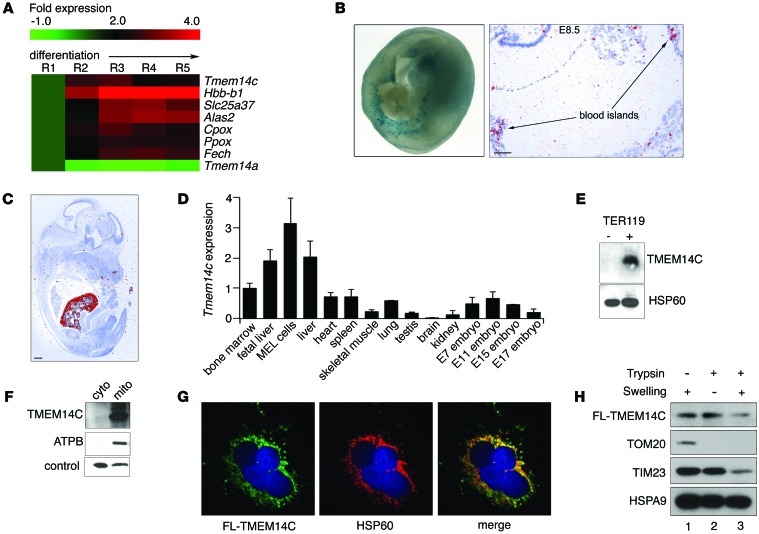
Figure 1. TMEM14C is enriched in differentiating murine erythroid cells and localizes to the inner mitochondrial membrane.
(A) RNAseq analysis of murine fetal liver cells sorted into 5 progressively differentiated erythroid subpopulations (R1–R5) shows that Tmem14c is upregulated during erythroid differentiation. (B) Tmem14c mRNA is expressed in hematopoietic organs, as shown by β-galactosidase staining (blue) of Tmem14c LacZ reporter expression in an E10.5 murine yolk sac (original magnification, ×63) and in situ hybridization of an E8.5 yolk sac (scale bar: 100 μm) and (C) fetal liver at E15.5 (pseudo-red; scale bar: 500 μm). (D) qRT-PCR shows Tmem14c mRNA is highly expressed in erythropoietic tissues and a MEL cell line. Tmem14c expression was normalized to Hprt levels. (E) Western blot analysis shows specific expression of TMEM14C protein in differentiating TER119+ murine fetal liver erythroid cells. HSP60 serves as a loading control. (F) Western blot analysis of fractionated Tmem14c-transfected HEK293T cells demonstrates localization of TMEM14C to the mitochondria. The control band indicates a protein that nonspecifically cross-reacts with MFRN1 antibody and migrates at a different molecular weight. (G) Confocal immunofluorescence microscopy (original magnification, ×63) shows that most of the transiently transfected FLAG-TMEM14C (fluorescein) colocalizes (merged, yellow) with HSP60 (rhodamine), a mitochondrial resident protein; nuclei were stained with DAPI (blue). (H) Transiently transfected FLAG-TMEM14C localizes to the inner mitochondrial membrane. TMEM14C, like TIM23, an inner mitochondrial protein, is sensitive to trypsin digestion when the outer mitochondrial membrane is disrupted by hypotonic swelling. The residual FLAG-TMEM14C that is trypsin resistant reflects mitochondria that are resistant to osmotic shock.