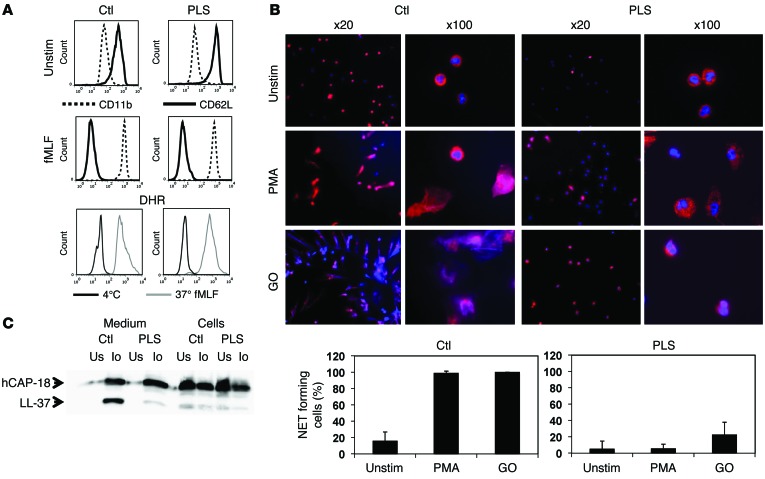
Figure 2. Functional studies on PLS neutrophils.
(A) Flow cytometry of nonstimulated and fMLF-stimulated (10–8 M) neutrophils from PLS and a normal control demonstrating equal disappearance of CD62L and upregulation of CD11b in response to stimulation. Respiratory burst was quantified as DHR-positive cells by flow cytometry of isolated neutrophils stimulated by fMLF (10–8 M). (B) NETosis. Neutrophils were stimulated with PMA or glucose oxidase (GO) to undergo NETosis and stained with DAPI and antibodies against MPO. NETosis was quantified as an increase in MPO-positive areas normally caused by formation of NETs with bound MPO. While some PLS neutrophils had increased MPO-positive areas after stimulation with glucose oxidase, these cells did not form NETs. The increased MPO-positive areas in these cells were caused by the presence of MPO in decondensated nuclei, as seen in the panels with ×100 magnification. Error bars for the PLS neutrophils demonstrate SD between the 2 experiments performed. One representative experiment out of 3 is shown for quantification of NETosis from the normal controls, and here error bars indicate SD between the 20 images used for quantification. (C) Processing of hCAP-18 by ionomycin-stimulated neutrophils as described in Methods. Cells and medium from ionomycin-stimulated (Io) or unstimulated neutrophils (Us) were blotted with a rabbit antibody against hCAP-18 (34). These functional studies (except the respiratory burst experiment, which was performed only once) were performed on 2 independent occasions 3 months apart with similar results.