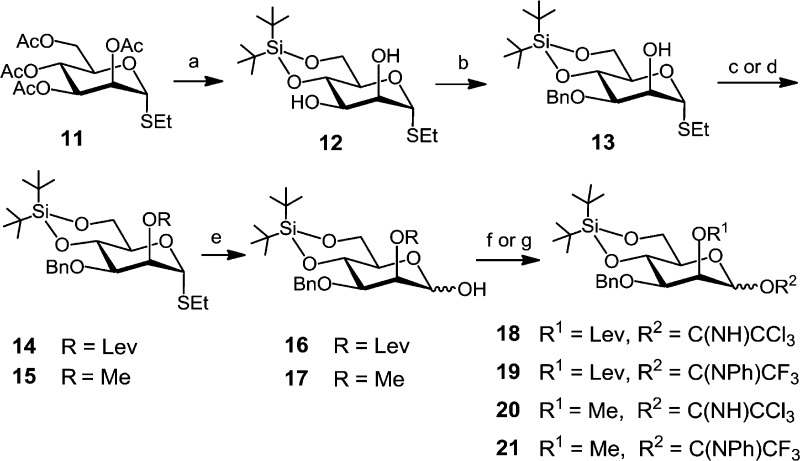
Scheme 3. Synthesis of Mannose-Based Donors.
Reagents and conditions: (a) 1. NaOMe, MeOH, 2. (tBu)2Si(OTf)2, pyridine, DMF, −35 °C, 89%; (b) 1. (nBu)2SnO, toluene, 2. BnBr, (nBu)4NI, DMF, toluene, reflux, 93%; (c) →14: LevOH, DIC, DMAP, CH2Cl2, 90%; (d) →15: MeI, NaH, DMF, 84%; (e) NBS, acetone–H2O, 24:1, 0 °C, 77% for 16 and 89% for 17; (f) →18 and 20: CCl3CN, DBU, CH2Cl2, 0 °C, 90% for 18 and 94% for 20; (g) →19 and 21: CF3(NPh)CCl, K2CO3, acetone, 99% for 19 and 93% for 21.