Figure 2. Potential endpoints for the treatment of sarcoidosis.
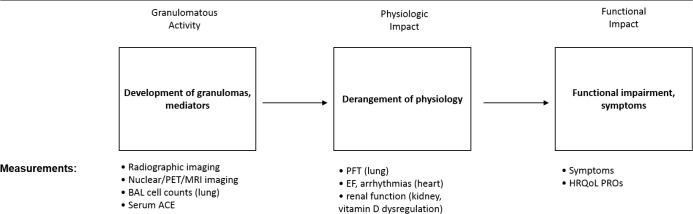
The granulomatous inflammation of sarcoidosis can be measured by various methods (left box).The endpoint of granulomatous inflammation would be appropriate to detect the anti-sarcoidosis activity of a drug. However, granulomatous inflammation from sarcoidosis may not result in physiologic impairment (middle box). When physiologic impairment occurs, it may be mild and not lead to the development of symptoms. Therefore, the presence of active sarcoidosis (left box) or physiologic impairment (middle box) may be inadequate clinical endpoints to base decisions on whether sarcoidosis patients require therapy. If physiologic impairment leads to significant functional impairment and/or worsening quality of life (right box), then therapy for sarcoidosis is required. Therefore, a rational clinical endpoint would need to incorporate the patient‘s functional status and health-related quality of life.
Abbreviations: ACE, angiotensin converting enzyme; BAL, bronchoalveolar lavage; EF, ejection fraction; HRQoL, health-related quality of life; MRI, magnetic resonance imaging; PET, positron emission tomography; PFT, pulmonary function test; PRO, patient reported outcome measure. Adapted from [80].
