Figure 4.
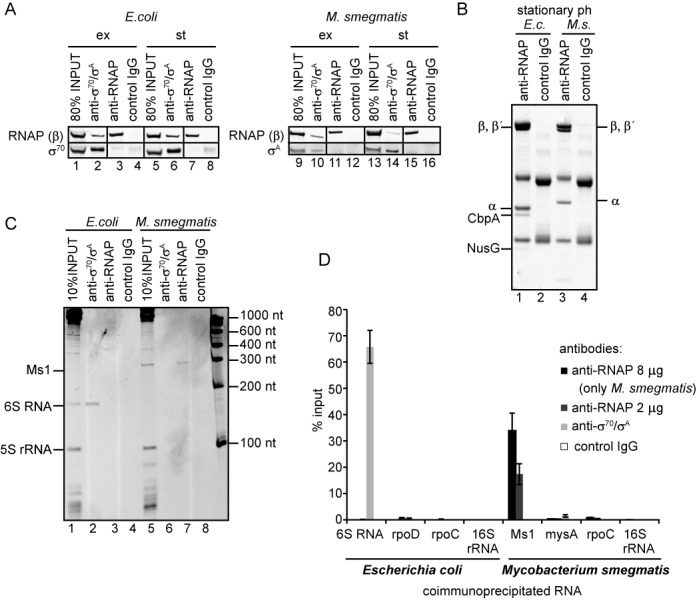
Ms1 interacts with core RNA polymerase. (A) Antibodies against RNAP (anti-RNAP; clone name 8RB13-it recognizes the core form of RNAP) and σA/σ70 (anti-σA/σ70, clone name 2G10-it recognizes also the holoenzyme containing σA/σ70) efficiently immunoprecipitated proteins from both Mycobacterium smegmatis and Escherichia coli. Immunoprecipitated proteins were visualized by western blotting. ex: exponential phase; st: stationary phase. ‘Control IgG’ is a mouse nonspecific IgG used as a negative control. The experiment was repeated 3× with identical results. (B) The RNAP antibody (8RB13) immunoprecipitated the core form of RNA polymerase. Immunoprecipitated proteins from E. coli (E.c.) and M. smegmatis (M.s.) stationary phase lysates were separated by SDS-PAGE and stained with Coomassie. ‘Control IgG’ stands for a mouse nonspecific IgG used as a negative control. Additional E. coli proteins were identified by mass spectrometry (see Supplementary Table S4). No σ factors were detected. No significant bands besides RNAP subunits were found for M. smegmatis. (C) RNAs coimmunoprecipitated with RNAP (2 μg of antibody) and σA/σ70 antibodies were resolved on PAGE and stained with GelRed or (D) quantified by RT-qPCR and normalized to the input. In immunoprecipitations quantified by RT-qPCR, two amounts of the anti-RNAP antibody (8RB13) were used (2 and 8 μg); the amount of coimmunoprecipitated Ms1 increased with the increased amount of the RNAP antibody, suggesting that the concentration of the RNAP antibody was not saturating. E. coli 6S RNA coimmunoprecipitated with σ70 which is in complex with RNA polymerase. None of the control mRNAs-two mRNAs: rpoD (σ70 mRNA), mysA (σA mRNA), rpoC (RNAP β′ subunit mRNA) and 16S rRNA were coimmunoprecipitated with the antibodies used. ‘Control IgG’ is mouse nonspecific IgG used as a negative control. Error bars are SEM (standard error of the mean) from at least three independent experiments.
