Figure 3.
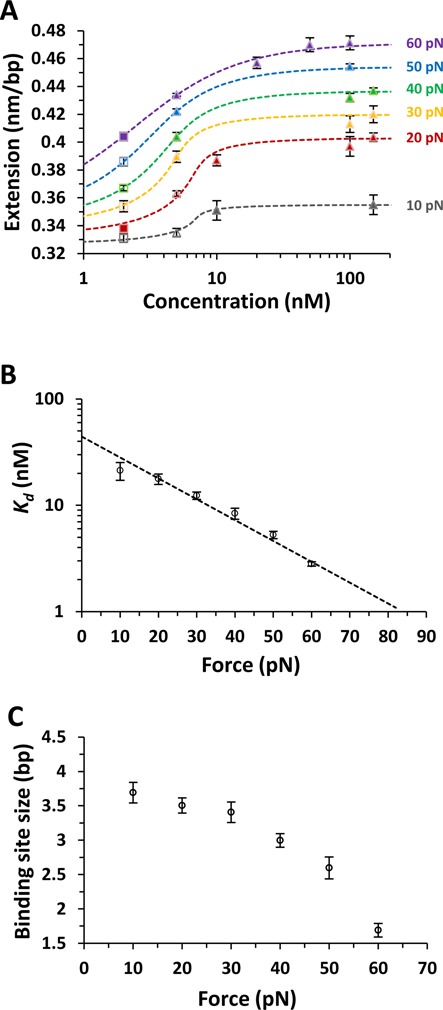
(A) Measured equilibrium extensions as a function of concentration fitted to the McGhee–von Hippel binding isotherm, yielding the dissociation constant (Kd) and the binding site size (n) for a range of applied forces between 10 pN and 60 pN. The dashed lines are the fits at each constant force as color coded, closed triangles are measurements, filled squares are previously reported measurements at 2 nM (10) and unfilled marks are obtained from the DNA-ligand complex WLC fit (Equation (3)). (B) Measured equilibrium dissociation constant Kd(F) (symbols) along with the fit to an exponential dependence on force (Equation 5), dashed line). The fit allows us to determine the zero force binding constant Kd(0) = 44±2 nM and the change in DNA length after equilibrium binding Δxeq = 0.19±0.01 nm. (C) The binding site size obtained from the McGhee–von Hippel binding isotherm ranging from 3.7±0.2 at 10 pN to 1.7±0.1 at 60 pN.
