Fig. 5.
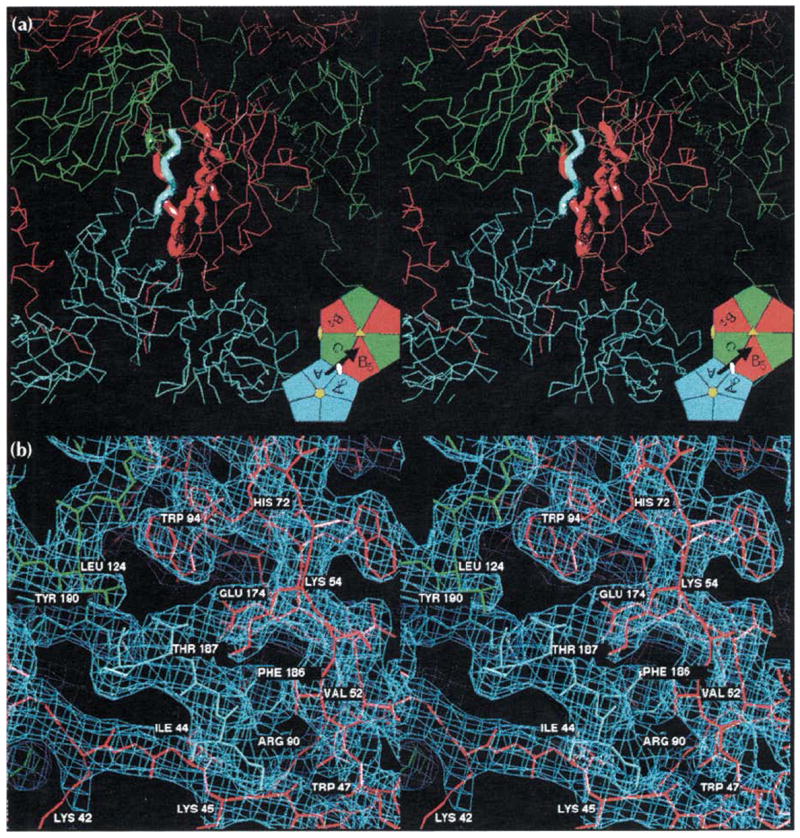
Stereoviews of the A-B5 quasi two-fold dimer contact responsible for binding pentamers to hexamers (see Fig. 1 for definitions and color coding). (a) An overview of the interaction showing the A subunits (blue) clustered around a five-fold axis and the B (red) and C (green) subunits clustered around a quasi six-fold axis. The bold tubes correspond to the regions of modeled electron density detailed in part (b). The inset is taken directly from Fig. 1b and displays the figure orientation relative to the particle symmetry axes. The black arrow represents the A subunit carboxyl terminus shown invading the B5 subunit here, and the visual orientation for (b). (b) A detailed view of the 3.2 Å electron density (light blue cage) for the interaction of the carboxy-terminal portion of the A subunit (blue wire model, residues 184–190) with the ‘clamp’ region of the B5 subunit (red wire model, residues 41–56, 91–95, 133–136, 171–176, the orientation is similar to that of the CCMV ribbon model in Fig. 2a). A small portion of the C subunit (green wire model, residues 123–125) is also visible. Hydrogen bonding of Thr187 by Glu174, the ‘fist in hand’ interactions of Phe186 (see text; Table 1), and the interaction of Leu124 of the quasi six-fold or five-fold related subunits with the Thr187 methyl group occur at each of the 180 clamp sites. This view is rotated 30° about the horizontal axis relative to (a). Views of the C-C2 icosahedral dimer contacts would be indistinguishable from those shown here except for the replacement of the pentamer with a second hexamer.
