Fig. 8.
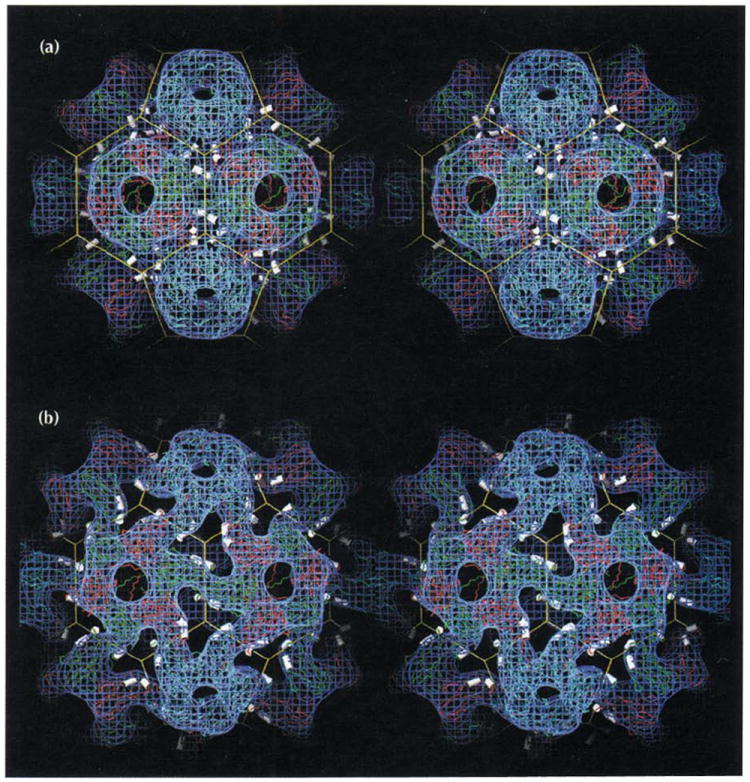
Stereoviews of two forms of the CCMV capsid showing that its polymorphism is controlled by changes in pH and metal ion concentrations. Atomic coordinates determined from X-ray crystallography and the truncated icosahedron cage are displayed as in Fig. 1a with electron density (blue) determined by cryo-electron microscopy. The helices which carry calcium-binding residues (αCDII and αGH in Figs 2a, 3, and 6) are displayed as white cylinders. (a) The CCMV capsid at pH 4.5. The atomic model is displayed as originally built in the X-ray electron density. The excellent fit between the 3.2 Å X-ray model and the 23 Å EM reconstruction shows the level of compatibility between the two independent structure determination techniques. Note the three pairs of CDII and GH helices positioned around each quasi three-fold axis (vertices of the truncated icosahedron). Calcium ions (180 per capsid) are bound between morphological units by residues associated with the helices (Table 2, Fig. 6). (b) The swollen CCMV capsid at pH 7.5 in the absence of metal ions determined at 28 Å resolution. Atomic coordinates from the high resolution native structure were modeled to fit the density from the cryoEM reconstruction.
