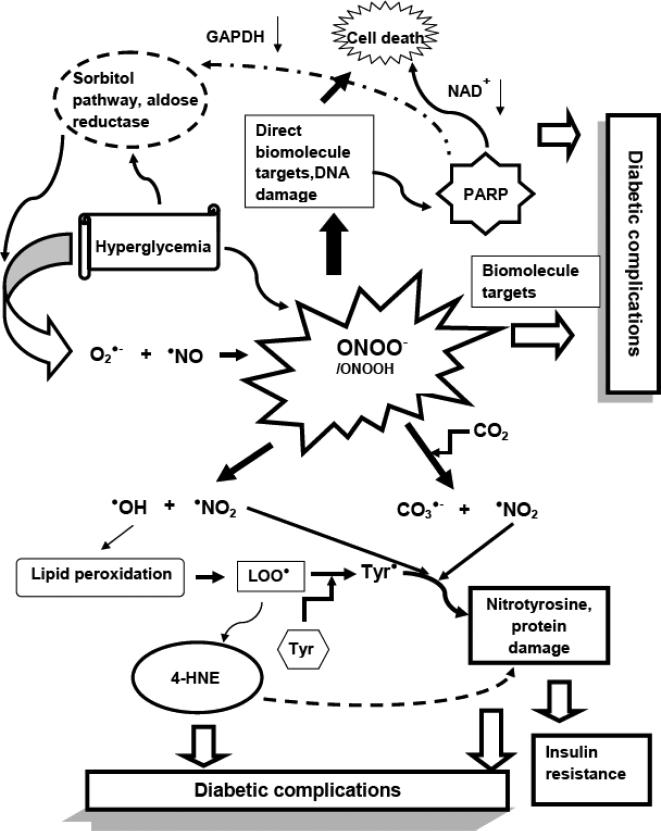
Fig. (2).
A short summary of peroxynitrite-mediated reactions and their possible relation to diabetic complications. Superoxide anion and nitric oxide reacts in a diffusion controlled fashion to form the potent oxidant peroxynitrite. The sources of superoxide in hyperglycemia can be several, including the activation of the polyol pathway and the enzyme aldose reductase as a major mechanism; mitochondrial oxidative stress, NADPH oxidases, uncoupled NOS and several others. NO levels can be elevated due to chronic low grade inflammation. Peroxynitrite affects biomolecules either directly, or by its homolytic cleavage or through its reaction with the ubiquitous CO2. When DNA is targeted, causing single strand breaks, it activates PARP. PARP activation leads to loss of NAD+ and ATP and eventually necrosis and cell death, contributing to diabetic pathogenesis or complications. From the various reactions of peroxynitrite, radicals can initiate lipid peroxidation, leading to the accumulation of lipid peroxide radicals (LOO•) and the toxic end product 4-hydroxynonenal (4-HNE). 4-hydroxynonenal has the ability to covalently bind to proteins and enzymes with vital role, interfering with their function. According to novel findings, lipid peroxide radicals may mediate the oxidation of tyrosine to Tyr radicals (Tyr•) in hydrophobic environments. The tyrosyl radical then can react with •NO2 to form nitrotyrosine, contributing to protein oxidation in tissue sites of diabetic complications.