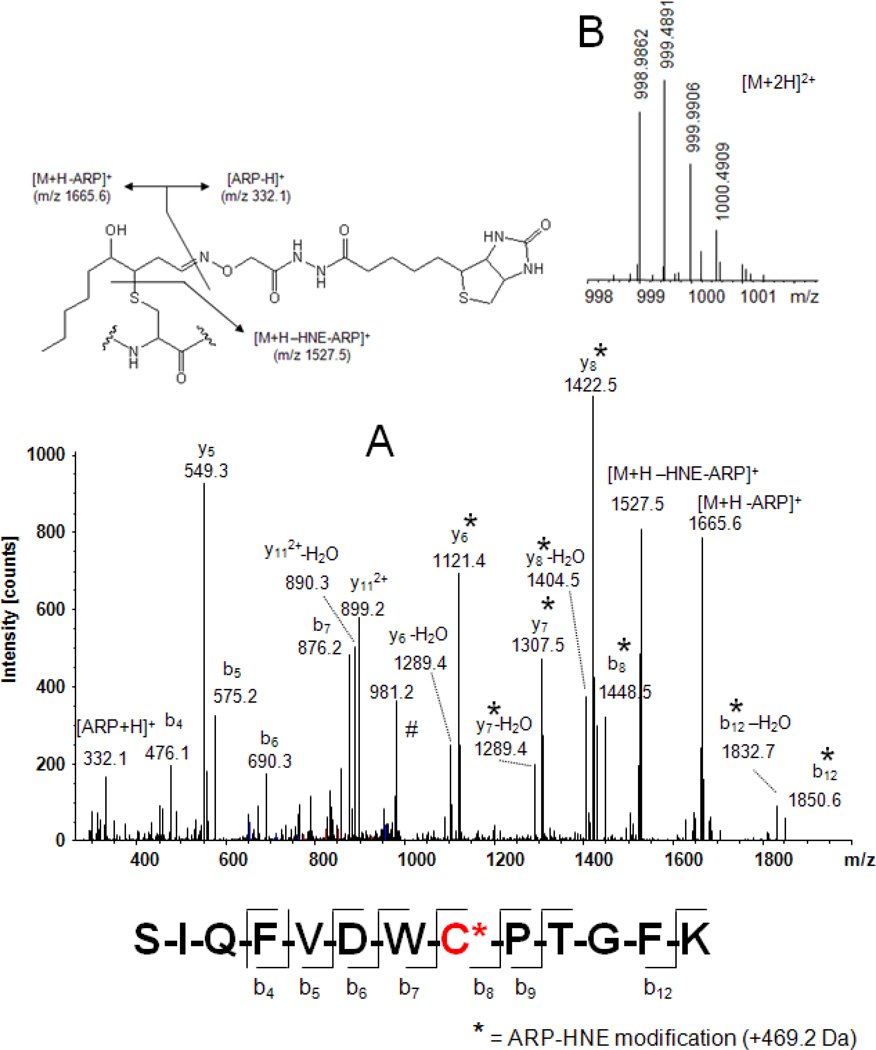
Fig. 6.
Mass spectral analysis of the ARP-labeled HNE-modified peptide SIQFVDWC*PTGFK from tubulin α-1B using a LTQ-FT mass spectrometer. A) Tandem mass spectrum of the doubly protonated precursor ion which was used for collision-induced fragmentation in the linear ion trap of an LTQ-FT mass spectrometer. Fragment ions marked with an asterisk (*) retained the ARP-HNE moiety during collision-induced fragmentation and allowed the unambiguous assignment of the Cys residue as site of HNE adduction. B) FT-ICR full scan mass spectrum showing the doubly protonated [M+2H]2+ ion cluster region. Exact mass determination using the ICR cell of the instrument yielded for the monoisotopic ion an m/z value of 998.9862 Th which reflects a mass accuracy of −0.4 ppm (calculated m/z 998.9866). Having both analytical information, sequencing data and exact mass, enables the identification of the peptide as the partial sequence 340–352 of tubulin α-1B chain (TBA1B_Human; Swiss-Prot P68363) with Cys-347 modified by HNE with high confidence.