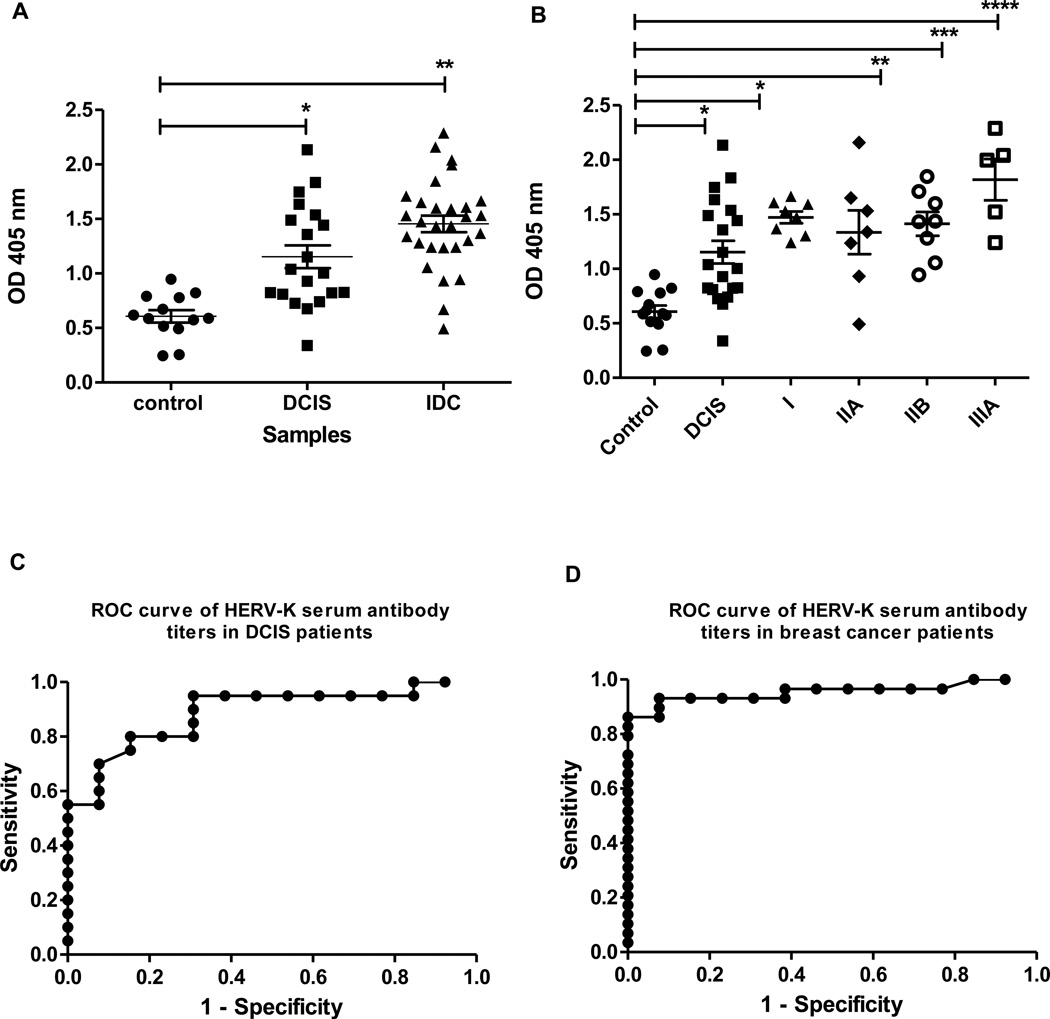
Figure 1.
Serum antibody titers in patients at various stages of breast cancer. A, Anti-HERV-K serum antibody titers in ductal carcinoma in situ (DCIS) and invasive ductal carcinoma (IDC). Sera from patients were diluted 1:100 and assayed for the presence of serum anti-HERV-K antibodies by ELISA. The antigen used for detection was the HERV-K env surface fusion protein. DCIS and IDC were significantly different from controls by the Wilcoxon rank-sum test (*, p = 0.0002; **, p < 0.0001). N=20 for DCIS, N=29 for IDC and N=13 for control. B, Anti-HERV-K serum antibody titers, analyzed by breast cancer stage. The differences between stages of breast cancer and controls were significant by the Wilcoxon rank-sum test (*, p = 0.0002; **, p = 0.079; ***, p = 0.0003; ****, p = 0.0016). N=20 for DCIS, N=8 for stage I, N=7 for stage IIA, N=8 for stage IIB, N=5 for stage IIIA and N=13 for control. Results in A and B are presented as individual data points for each subject. C, ROC curve of HERV-K serum antibody titers in DCIS patients. D, ROC curve of HERV-K serum antibody titers in breast cancer patients.