Figure Four. Vinculin stabilizes focal adhesions to facilitate FAK and PI3K/Akt signaling, drivers of cellular invasion in 3D.
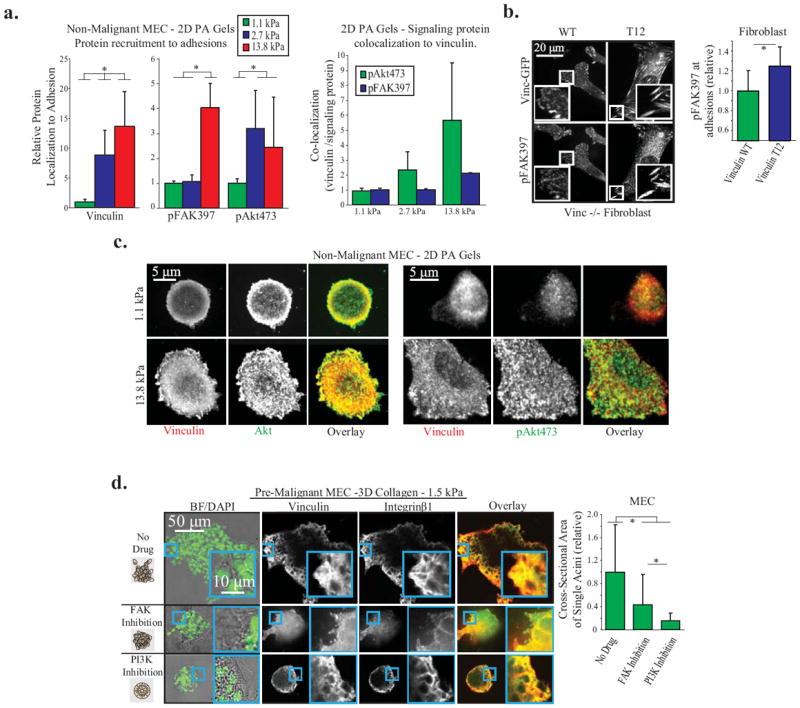
A. Vinculin, p473Akt, and p397FAK recruitment to adhesions in non-malignant MEC on 2D PA Gels of different stiffness, coated with fibronectin. Left: Protein recruitment measured by quantitative immunofluorescence at single pixels (0.011 μm2) and averaged over whole cell (p < .01; ±SD, n>18 cells per condition) Right: Quantification of signaling protein colocalization to vinculin per pixel cell, averaged over whole cell (p < .01; ±SD, n>18 cells per condition). B. p397FAK recruitment to adhesions in vinculin homozygous null mouse fibroblasts expressing vinculin WT or T12 plated on a 2D PA gel. Vinculin T12 significantly increases p397FAK; measurements made at single pixels and averaged over whole cell (p < .05; ±SD, n>9 cells per condition). C. Vinculin, Akt, and p473Akt recruitment to adhesions in non-malignant MEC on 2D PA Gels. Akt is recruited to adhesions on soft and stiff gels, while p473Akt is dramatically increased in adhesions on stiff PA gels. D. Pre-malignant MEC spheroids in 1.5 kPa 3D collagen gels for 48 hours, with FAK (1μM FAK Inhibitor 14) or PI3K (1μM GDC-0941) inhibition. Before fixation, the cross-sectional area of spheroids was measured to quantify 3D cell invasion (p < .01; ±SD, >26 spheroid per condition). Spheroids were then cryosectioned and immunostained for vinculin and integrin β1 to demonstrate that focal adhesions are not ablated after PI3K or FAK inhibition.
