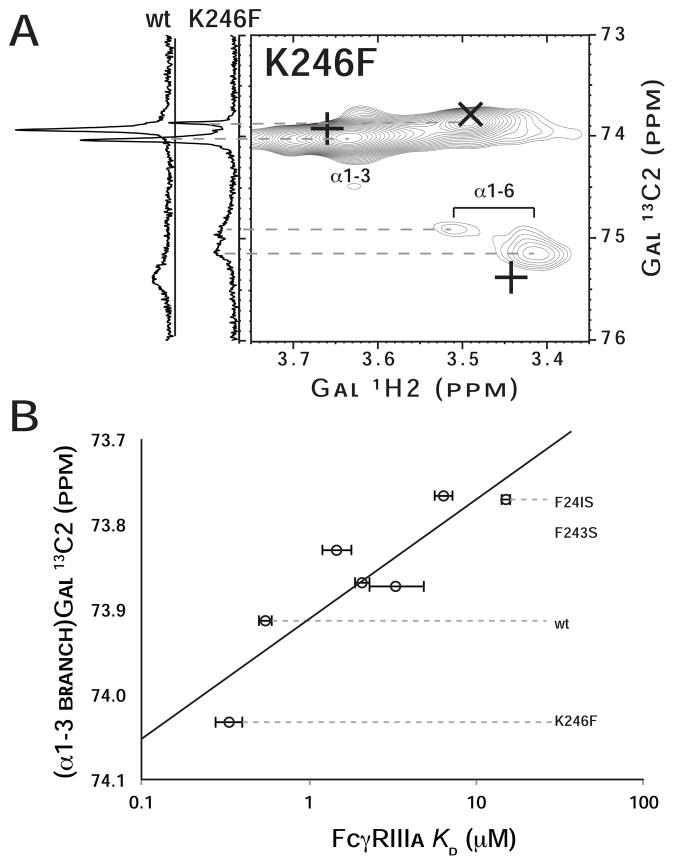
Figure 6. A mutation designed to enhance the interaction between the glycan and polypeptide increases affinity for Fc.
γRIIIa.
(A) An 1H-13C HSQC spectrum of K246F mutant of Fc at 50°C containing the G2F glycan with 13C2-enriched Gal termini is shown. Positions of wild type coherences are highlighted in each spectrum with “+” signs, and the position of coherences following trypsin proteolysis are marked with “x”. To the left of the 2d HSQC spectrum, 1d 13C spectra of wt at K246F glycans are shown. (B) The resonance frequency of (α1–3 branch)Gal 13C2 nuclei correlates with FcγRIIIa affinity (R2=0.79).