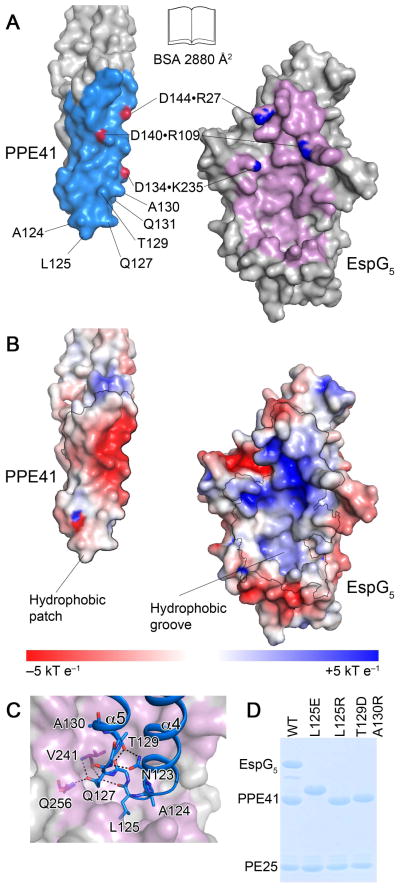
Fig. 3. The interface between EspG5 and PPE41.
A. An ‘open book’ view of the PPE41–EspG5 complex. Contact residues in the interface are colored in light blue (PPE41) and purple (EspG5). Atoms participating in intermolecular salt bridges are colored in red and blue.
B. EspG5 and PPE41 are shown in the same orientation as in panel (A). The surface is colored according to electrostatic surface potential contoured at ±5 kT e−1, with red corresponding to a negative and blue to a positive potential. The contact areas are indicated by black lines.
C. EspG5 is shown in surface representation as in panel (B), PPE41 is shown in ribbon representation (blue). Residues in α4-α5 loop are shown in stick representation. Hydrogen bonds are shown as black dashed lines.
D. SDS-PAGE analysis of co-purification of EspG5 and PE25–PPE41 mutant variant dimers. Proteins were purified by affinity chromatography from the lysate of E. coli strain expressing N-terminally His-tagged PE25, PPE41 and EspG5. Note that PPE41L125E has an altered mobility on SDS-PAGE.