Abstract
We present here three cases of plasma cell dyscrasias; first case presenting as primary plasma cell leukemia showing unusual morphology and aberrant expression of myeloid markers; the second case presenting as plasma cell leukaemia with atypical plasma cells in peripheral blood and the third case presenting as myelomatous pleural effusion after treatment for myeloma.
Keywords: Plasma cell leukemia, Aberrant expression, Myeolomatous pleural effusion
Introduction
Plasma cell leukemia (PCL) is a rare and aggressive variant of multiple myeloma with poor prognosis characterized by peripheral blood involvement. The diagnostic criteria of PCL are (1) blood leukocyte count exceeding 10 × 109/L, with at least 2 × 109/L plasma cells, or (2) for a peripheral blood leukocyte count below 10 × 109/L, at least 20 % of the circulating cells must be plasma cells [1–3]. Rarely PCL with aberrant morphologic features like cleaved, monocytoid or multi-lobulated nuclei has been reported [1, 4, 5]. These morphologic characteristics have a poorer prognosis than the usual well-differentiated myeloma and are confused with myeloid or monocytic leukemias and lymphomas. Pleural effusion is a rare presenting feature of multiple myeloma [6–8]. We present three cases of PCL with unusual presentations—first case of primary PCL with aberrant myeloid markers, second case of PCL with aggressive clinical presentation and third case of multiple myeloma which progressed to PCL with myelomatous pleural effusion.
First Case
A 51 year lady presented with anasarca of 3 months duration with loss of 10 kg of weight in last 1 year. Her co-morbidities included diabetes mellitus, hypothyroidism, hyperlipidemia and pulmonary arterial hypertension with cor pulmonale. On examination, she was febrile with a vague mass felt per abdomen. Her haemoglobin was 7.7 g/dL, total leucocyte count 14.9 K/cmm and platelet 180 K/cmm. Serum electrophoresis was within normal limits. No M band or light chains were found.
Serum LDH was 864.0 U/L. Beta-2 microglobulin was 10.5 mg/L. Liver function tests were within normal limits except serum bilirubin which was 3.6 mg/dL. Chest X ray was normal. ECHO cardiogram showed right ventricular and right atrial enlargement, small pericardial effusion and cor pulmonale.
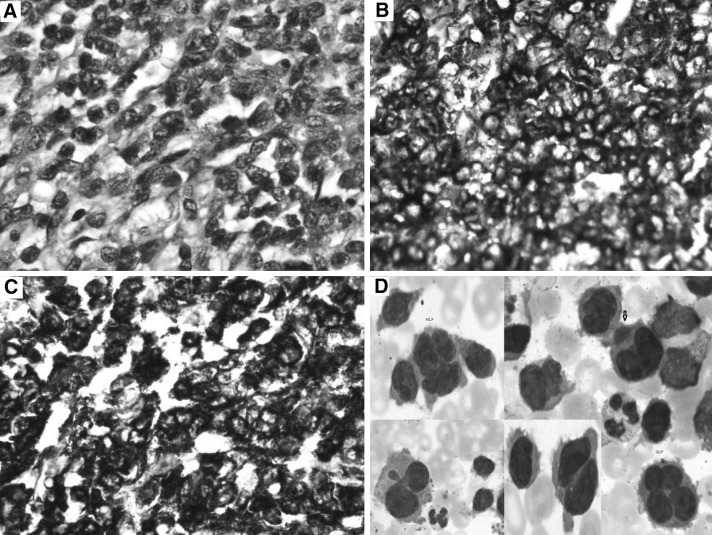
Manual differential count (DC) showed 30 % atypical cells in peripheral smear. Bone marrow aspiration showed hypercellular marrow with many atypical cells. The cells were of varying sizes with moderate to abundant basophilic cytoplasm with cytoplasmic protrusions and prominent nucleoli in some. Atypical bi-to-multinucleated and multi-lobulated cells were seen (Fig. 1d). Bone marrow biopsy showed hypercellular marrow with sheets of atypical cells (Fig. 1a). Immunohistochemistry revealed focal positivity for epithelial membrane antigen, strong membrane positivity for CD-138 (Fig. 1c) and CD-43 (Fig. 1b). IHC for LCA, CD20, CD3, TdT, C-Kit, CD31, CD34, CD56 and MPO were negative. Proliferative activity was increased focally. Flow-cytometry showed 30 % atypical cells with CD13, CD31 and CD43 positivity in more than 90 % cells. MPO and HLA-DR were negative. PET-CT showed multiple ametabolic lytic lesions in skull, atelectasis in basal segment of left lower lobe, nodularity of left lateral pelvic wall, erosions of S1–S2 vertebral bodies, gross S3–S4 involvement with soft tissue component and left second rib involvement. A diagnosis of acute plasma cell leukaemia with abnormal morphology and aberrant myeloid marker was made. She was planned for bortezomib/lenalidomide regime. She was reviewed after nine injections of bortezomib and she was in good condition. Follow-up peripheral smear showed 15 % plasma cells.
Fig. 1.
a H&E×40. Bone marrow biopsy showing atypical plasma cells. b IHC ×40. Bone marrow showing CD 43 positivity. c IHC ×40. Bone marrow biopsy showing CD 138 positivity. d Leishman’s stain ×100. Bone marrow smear showing atypical plasma cells with unusual morphology. Binucleated, multinucleated and multi lobulated plasma cells are seen
Second Case
A 52 year old male patient was evaluated for bilateral lower limb swelling, breathing difficulty and anaemia of 3 months duration. He had bilateral deep vein thrombosis with pulmonary embolism, bilateral pneumonia with acute respiratory failure, uncontrolled diabetes mellitus type 2. Haemogram showed haemoglobin of 8 g/dL, TLC 18.9 K/cmm, platelet 280 K/cmm and ESR 140 mm/h. X-ray of pelvis and lumber spines showed decreased bone density and dystrophic calcification of left gluteal region near greater trochanter of femur.
Manual DC showed 58 % atypical plasma cells (Fig. 2b). He had prominent M band while M component was 3.5 g/dL. Bone-marrow aspirate showed 90 % atypical plasma cells (Fig. 2a). Bence Jones proteins were not seen in urine. Patient was started on IV antibiotics and other supportive measures for acute respiratory infection. Sputum culture grew Candida. Hence antifungal started. During this treatment, he developed generalized tonic–clonic seizures and was started on antiepileptic measures. The prognosis was poor.
Fig. 2.
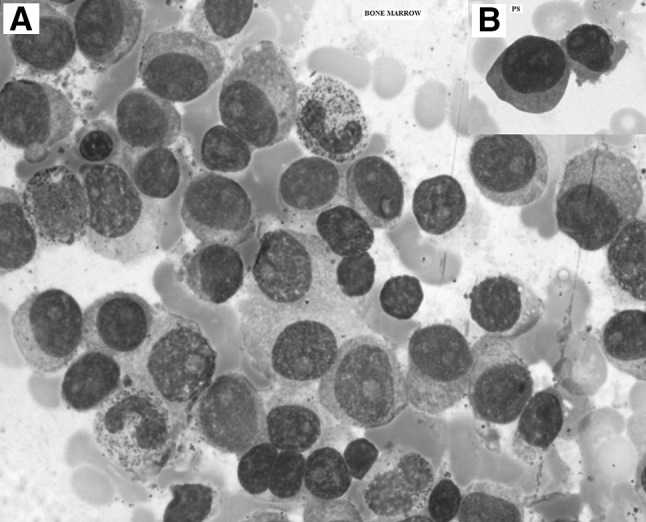
a Leishman’s stain ×100. Bone marrow smear showing atypical plasma cells with abundant basophilic cytoplasm and prominent nucleoli. b Leishman’s smear ×100. Peripheral smear shows abnormal plasma cells
Third Case
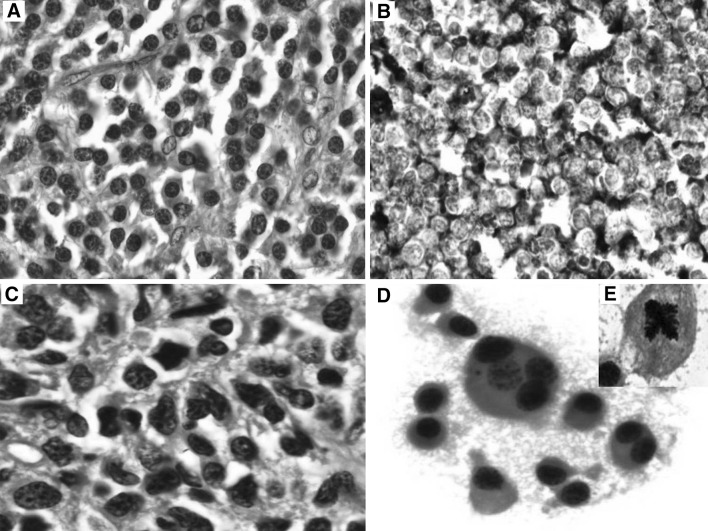
A 44 year old male patient presented with chest wall mass of 4 months duration. Biopsy and IHC suggested plasmacytoma (Fig. 3a, b). Bone marrow aspiration and biopsy revealed multiple myeloma (Fig. 3c). Lytic lesions were seen in skull vault and in lower dorsal lumber vertebra. ESR was 90 mm/h and M Component was 0.9 g/L. Patient was diagnosed as multiple myeloma Stage III A. He was considered for autologous bone marrow transplant and hence started on Zometa injection, thalidomide and dexamethasone. After four cycles of chemotherapy, bone marrow showed residual disease. He underwent autologous stem cell transplant followed by six cycles of chemotherapy. After 9 months of stem cell transplant, he presented with pain and swelling in the left leg and was not able to walk. Biopsy revealed multiple myeloma. Bone marrow biopsy revealed residual disease. M component was negative. Radiation (30 grey) was delivered to left thigh. He was given three more cycles of thalidomide and dexamethasone. He was on regular follow up but again developed left leg pain after 8 months. Bone marrow showed 30 % atypical plasma cells. He was started on bortezomib and Dexamethasone. Patient developed prolonged neutropenia with low vitamin B-12. He was started on vit B-12 injection. He was planned for 2nd cycle of bortezomib at 50 % but he complained of chest pain. He had developed pleural effusion on left side. Pleural fluid cytology revealed sheets of atypical plasma cells suggestive of myelomatous effusion (Fig. 3d). He was diagnosed as having refractory disease and was advised palliative care with endoxan schedule.
Fig. 3.
a H&E ×40: chest wall tumour showing abnormal plasma cells. b IHC ×40: abnormal plasma cells showing CD 138 positivity. c H&E ×100: bone marrow biopsy showing atypical plasma cells. d Pap smear ×100: pleural fluid showing abnormal plasma cells. Bi and multinucleated forms are seen. Inset shows abnormal (tetrapolar) mitosis
Discussion
Plasma cell leukemia can either originate de-novo (primary PCL) or as secondary leukemic transformation of multiple myeloma (secondary PCL) [2]. PCL is associated with a poor prognosis, with a shorter survival than that of patients presenting with typical multiple myeloma. It has distinctive clinical, immunophenotypic and genotypic abnormalities as compared to multiple myeloma. Patients with PCL have higher prevalence of hepatosplenomegaly, lymphadenopathy and thrombocytopenia. They also have a lower serum M protein level, extramedullary involvement, and frequently presents with renal failure [2, 9–11]. The surface phenotype of plasma cells in PCL is different from that of bone marrow plasma cells in patients with multiple myeloma: CD9, HLADR, CD117, and CD20 are differentially expressed in circulating plasma cells compared with bone marrow plasma cells in myeloma patients.
Our first patient presented with atypical cells in peripheral blood and bone marrow with abnormal morphology with cleaved, bi to multinucleated and multilobated cells mimicking other haematological malignancies like lymphomas and myeloid leukaemias [1, 2, 4]. The presence of the myeloid antigen CD13 on myeloma cells is an uncommon finding [3]. Since CD13 antigen is expressed by cells of the granulocytic and monocytic lineages, its presence on plasma cells supports the hypothesis of an early stem cell disorder in the development of PCL. The patient was in good condition after initial botezomib/Lenalidomide regimen chemotherapy [8–10].
The second case of PCL had bilateral deep vein thrombosis with pulmonary embolism, bilateral pneumonia with acute respiratory failure and uncontrolled diabetes mellitus type 2. He was started on supportive care but developed status epilepticus indicating very poor prognosis.
The third patient was a known case of multiple myeloma and had autologous marrow transplant after initial four cycles of chemotherapy and had four more cycles of chemotherapy, but the disease recurred in left thigh with involvement of bone marrow. He was given 30 grey of radiotherapy to left thigh followed by chemotherapy but he developed prolonged neutropenia and left side pleural effusion. Pleura fluid cytology was diagnostic of myeloma. He was diagnosed having refractory disease and was advised palliative care with endoxan schedule.
In conclusion, PCL is a rare form of aggressive plasma cell dyscrasias and poses a diagnostic and therapeutic challenge. Aberrant morphologic features of cells like cleaved, monocytoid or multilobated nuclei may be confused with myeloid or monocytic leukemias lymphomas or nonhematologic malignancies. Meticulous laboratory investigations including immuno-histochemistry and flow-cytometry play important role in arriving at correct diagnosis.
Conflict of interest
None declared.
Contributor Information
Urmila Majhi, Phone: 044-22463376, Email: urmilamajhi@gmail.com.
Kanchan Murhekar, Email: kmurhekar@rediffmail.com.
Shirley Sundersingh, Email: shirleysundersing@hotmail.com.
K. R. Rajalekshmi, Email: rajalekshmykraghavan@yahoo.in
References
- 1.Panizo C, Cuesta B, Rocha E. Primary plasma cell leukemia with unusual morphology and complex karyotype. Haematologica. 1998;83:849–850. [PubMed] [Google Scholar]
- 2.Gertz MA, Buadi FK. Plasma cell leukemia. Haematologica. 2010;95:705–707. doi: 10.3324/haematol.2009.021618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernández de Larrea C, Kyle RA, et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia. 2013;27:780–791. doi: 10.1038/leu.2012.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zukerberg LR, Ferry JA, Conlon M, Harris NL. Plasma cell myeloma with cleaved, multilobated, and monocytoid nuclei. Am J Clin Pathol. 1990;93:657–661. doi: 10.1093/ajcp/93.5.657. [DOI] [PubMed] [Google Scholar]
- 5.Shin JS, Stopyra GA, Warhol MJ, Multhaupt HA. Plasmacytoma with aberrant expression of myeloid markers, T-cell markers, and cytokeratin. J Histochem Cytochem. 2001;49:791–792. doi: 10.1177/002215540104900613. [DOI] [PubMed] [Google Scholar]
- 6.Sher T, Miller KC, Deeb G, Lee K, Chanan-Khan A. Plasma cell leukaemia and other aggressive plasma cell malignancies. Br J Haematol. 2010;150:418–427. doi: 10.1111/j.1365-2141.2010.08157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YM, Lee KK, Oh HS, et al. Myelomatous effusion with poor response to chemotherapy. J Korean Med Sci. 2000;15:243–246. doi: 10.3346/jkms.2000.15.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirdel A, Attarn D, Ghobadi H, Ghiasi T. Myelomatous pleural effusion. Tanaffos. 2007;6:68–72. [Google Scholar]
- 9.Athanasiou E, Kaloutsi V, Kotoula V, et al. A morphologic, immunohistochemical, and in situ hybridization study of 71 paraffin-embedded bone marrow biopsy specimens. Am J Clin Pathol. 2001;116:535–542. doi: 10.1309/BVT4-YP41-LCV2-5GT0. [DOI] [PubMed] [Google Scholar]
- 10.Pagano L, Valentini CG, De Stefano V, et al. Primary plasma cell leukemia: a retrospective multicenter study of 73 patients. Ann Oncol. 2011;22:1628–1635. doi: 10.1093/annonc/mdq646. [DOI] [PubMed] [Google Scholar]
- 11.Colovic´ M, Jankovic´ G, Suvajdzic´ N, et al. Thirty patients with primary plasma cell leukemia: a single center experience. Med Oncol. 2008;25:154–160. doi: 10.1007/s12032-007-9011-5. [DOI] [PubMed] [Google Scholar]