FIGURE 2.
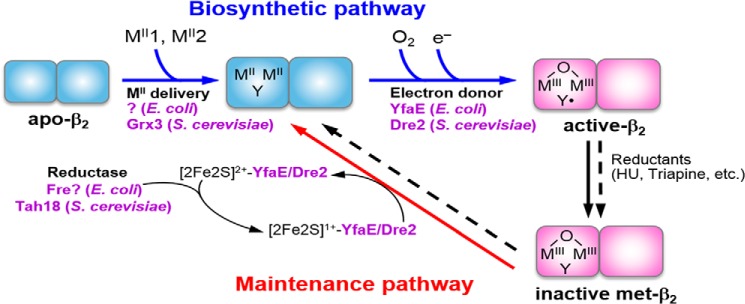
A model for the biosynthesis and maintenance pathways of the FeIII2-Y• cofactor of the class Ia RNR in E. coli and S. cerevisiae. Cofactor assembly in only one monomer of β2 is shown for simplicity. The biosynthetic pathway requires loading of two Fe2+ and one reducing equivalent per β to carry out the four-electron reduction of O2 to H2O (Reaction 2); the other three electrons come from the two Fe2+ and the Tyr residue that form the FeIII2-Y•. YfaE in E. coli and Dre2 in S. cerevisiae are proposed to supply the obligatory electron by oxidation of their [2Fe-2S]1+ to [2Fe-2S]2+, which is subsequently re-reduced to [2Fe-2S]1+ by Fre and Tah18, respectively. The maintenance pathway may use the same source of reducing equivalents to convert the inactive FeIII2-Y cluster to FeII2-Y, which subsequently reforms FeIII2-Y• in the presence of O2 via the biosynthetic pathway. HU = hydroxyurea.
