FIGURE 7.
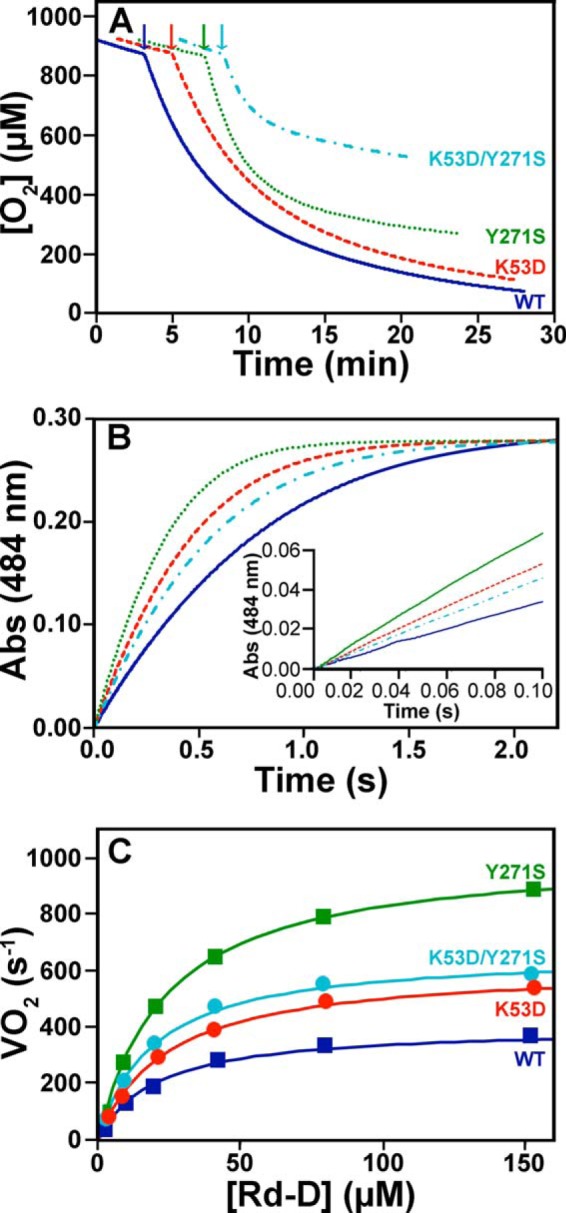
Reactivity of EhFdp1 variants with oxygen under multiple turnover conditions. Panel A, oxygraphic traces collected in O2-equilibrated buffer (50 mm Tris-HCl, 18% (v/v) glycerol, pH 7.5), in the presence of 5 mm NADH, 0.5 μm FlRd-Red, 2.5 μm Rd-D, 640 nm catalase, and 240 nm superoxide dismutase, following the addition of the EhFdp1 variants (50 to 120 nm). Panel B, Rd-D oxidation by EhFdp1 variants, with O2 as the terminal electron acceptor, as followed at 484 nm by stopped-flow absorption spectroscopy. Traces are shown for a single Rd-D concentration (40 μm). Rd-D was anaerobically pre-reduced with 400 μm NADH and 20 nm FlRd-Red in the presence of 1.3 μm catalase, and mixed at 25 °C with 100 nm EhFdp1 in air-equilibrated buffer. Data were fit with single exponential curves: solid blue line, WT; dashed red line, K53D; dotted green line, Y271S; dash-dotted cyan line, K53D/Y271S. Inset, zoom-in on the first 100 ms of the reaction for a better comparison of the initial rate of the reaction, among the tested EhFdp1 variants. Panel C, VO2 for WT and mutated EhFdp1 as measured at increasing concentrations of Rd-D.
