FIGURE 1.
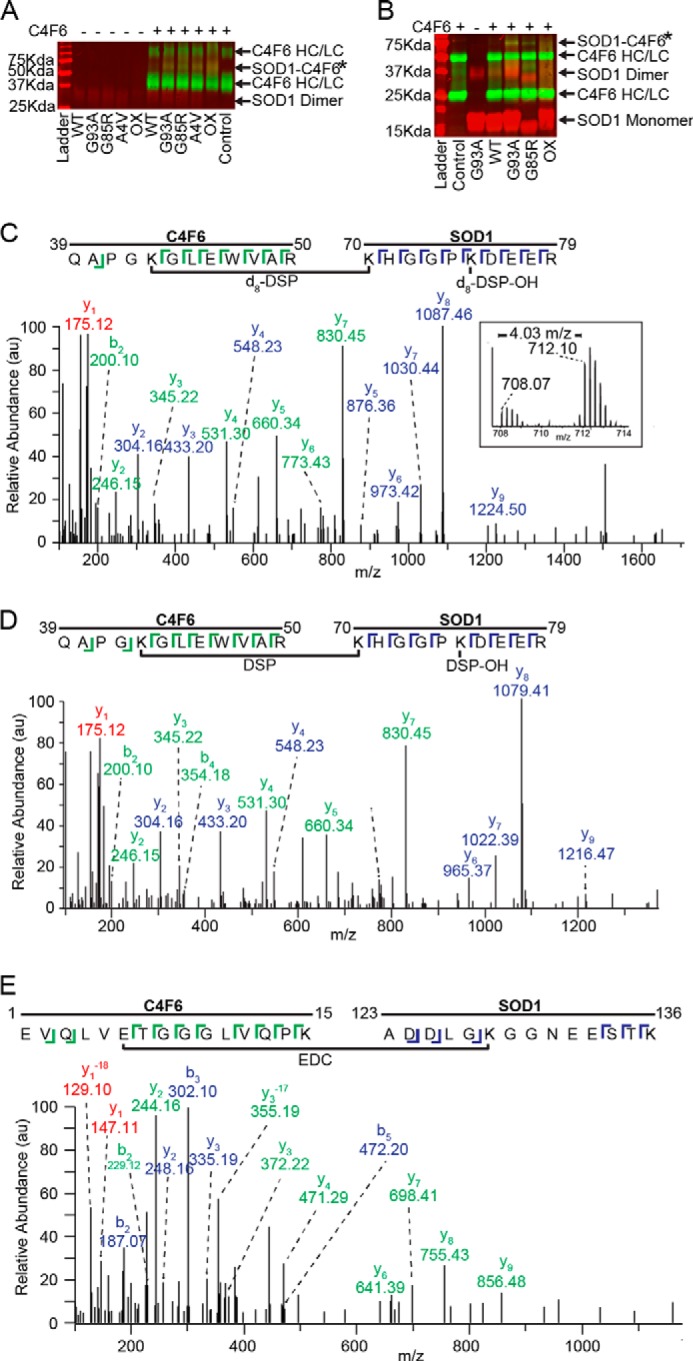
C4F6 specifically cross-links to misfolded SOD1 in the presence of DSP or EDC. A, a nonreducing Western analysis with pan-SOD1 (red) and anti-Fab (green) antibodies demonstrates the specific cross-linking with DSP between misfolded SOD1 variants and C4F6 Fab (SOD1-C4F6; yellow band at 66 kDa denoted by *). HC, heavy chain; LC, light chain. B, a band corresponding to the SOD1-C4F6 complex cross-linked with EDC (denoted by *) was detected at ∼70 kDa for SOD1 (G93A, G85R, and ox), but not WT SOD1. A similar SDS-PAGE and Western analysis was performed as in A, except a 12% gel was employed under reducing conditions. C and D, representative cross-linked peptides from a pooled (d8-DSP + DSP) sample where a 4.03 m/z shift was observed between the monoisotopic peaks (C, inset;16.13 Da mass shift/+4 charge state = 4.03 m/z) 708.07 m/z (DSP) and 712.10 m/z (d8-DSP). Both parent ions, 708.07 m/z (C, d8-DSP) and 712.10 m/z (D, DSP), were subjected to MS/MS analysis and identified as residues 70–79 of SOD1 and residues 39–50 of C4F6. E, exemplar MS/MS data (59% sequence coverage) for an EDC cross-linked peptide comprised of C4F6 (residues 1–15) and SOD1 G93A (residues 123–136). C–E, C4F6 (green) and SOD1 (blue) ions are depicted with the corresponding sequence displayed above. Peaks with identical ions in both C4F6 and SOD1 peptides are shown in red, where −18 corresponds to a loss of a water molecule for y1.
