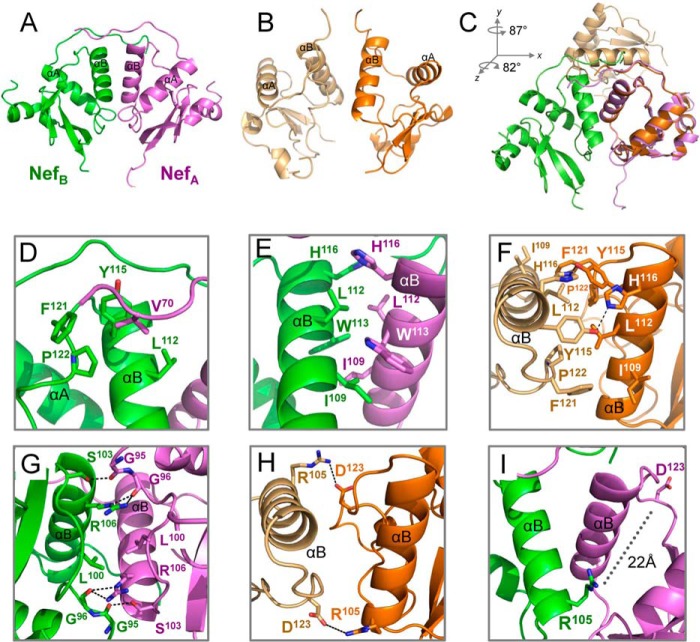
FIGURE 5.
Hck SH3-SH2 binding stabilizes a compact Nef dimer. Comparison of the Nef dimer in the Nef·Hck32 complex (A; Nef monomers are green and purple) with the Nef dimer from a previous Nef·SH3 complex (B; Nef monomers are light and dark orange; PDB code 1EFN (18)). C, superposition of Nef monomers from the structures in A and B. The resulting rotations along the y and z axes for the non-superimposed Nef monomer are shown. D, hydrophobic dimer interface of residue Val-70 with a pocket formed by αB-helix residues Leu-112 and Tyr-115 and C-terminal loop residues Phe-121 and Pro-122. E, hydrophobic dimer interface formed by interweaving of αB-helix residues Ile-109, Leu-112, Trp-113, and His-116. F, Nef residues Ile-109, Leu-112, Tyr-115, His-116, Phe-121, and Pro-122 also participate in the dimer interface of the Nef·SH3 structure. G, Nef dimer interface involving hydrophobic interactions between the side chains of residues Leu-100 and Arg-106 and a network of hydrogen-bonding contacts between the main-chain carbonyl atoms of Gly-95 and Gly-96 and side chains of Ser-103 and Arg-106. H, close up view of the dimer interface from a complex of Nef with an SH3 domain showing the role of Asp-123–Arg-105 interaction in dimerization. I, Nef Asp-123 is repositioned away from the dimer interface to the surface of the structure more than 22 Å away from Nef Arg-105 in the Nef·Hck32 structure. Arg-105 makes a new contact with Glu-93 in the SH3 domain in this reoriented dimer (see Fig. 6).