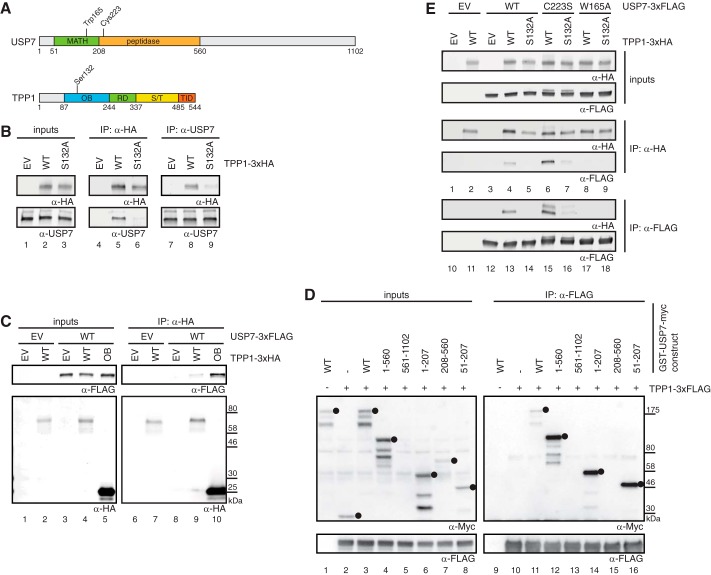
FIGURE 1.
TPP1 interacts with USP7. A, schematic depiction of USP7 and TPP1 domain organizations. RD, recruitment domain for interaction with POT1; S/T, Ser/Thr-rich domain; TID, TIN2 interaction domain. B, cells were transfected with the indicated TPP1 constructs. 48 h post-transfection, extracts were prepared (lanes 1–3) followed by anti-HA (lanes 4–6) or anti-USP7 (lanes 7–9) immunoprecipitation (IP) and Western blot analysis. TPP1(WT)-3xHA is co-immunoprecipitated with endogenous USP7, and the interaction is weakened by an S132A mutation in TPP1. C, cells were co-transfected with the indicated USP7 and TPP1 constructs. 48 h post-transfection, extracts were prepared (lanes 1–5) followed by anti-HA immunoprecipitation (lanes 6–10) and Western blot analysis. The OB domain of TPP1 is sufficient for the interaction with USP7. D, cells were co-transfected with the indicated USP7 and TPP1 constructs. 48 h post-transfection, extracts were prepared (lanes 1–8) followed by anti-FLAG immunoprecipitation (lanes 9–16) and Western blot analysis. Dots indicate the GST-tagged USP7 fragments. Note that fragment USP7(561–1102) was not expressed in cells. The MATH domain of USP7 mediates its interaction with TPP1. E, cells were co-transfected with the indicated USP7 and TPP1 constructs. 48 h post-transfection, extracts were prepared (inputs) followed by anti-HA (lanes 1–9) or -FLAG (lanes 10–18) immunoprecipitation and Western blot analysis. Compared with USP7(WT), USP7(C223S) shows a stronger interaction and USP7(W165A) shows no interaction with TPP1. EV, empty vector.