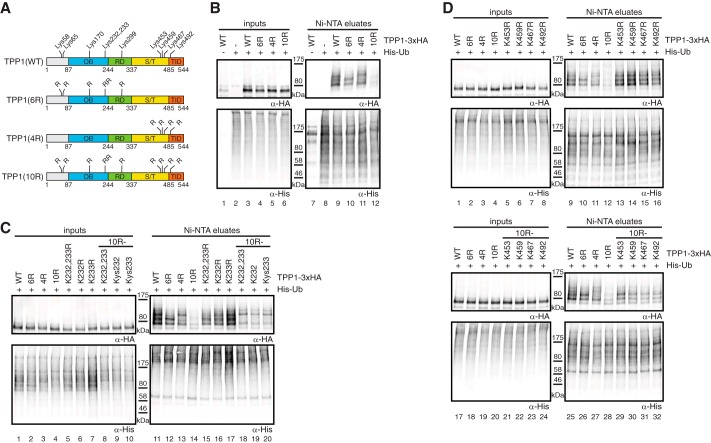
FIGURE 2.
TPP1 is ubiquitinated at several Lys residues. A, scheme indicating the 10 Lys residues of TPP1(WT) of which the six most N-terminal are mutated to Arg in TPP1(6R), the four most C-terminal are mutated in TPP1(4R), and all 10 are mutated in TPP1(10R). RD, recruitment domain for interaction with POT1; S/T, Ser/Thr-rich domain; TID, TIN2 interaction domain. B, cells were co-transfected with His-ubiquitin (Ub) and the indicated TPP1–3xHA constructs. 46 h post-transfection, cells were treated with 10 μm MG132. After 2 h, cells were harvested followed by urea extraction (lanes 1–6), Ni-NTA binding (lanes 7–12), and Western blot analysis. TPP1–3xHA is ubiquitinated, and its ubiquitination pattern changes for both the TPP1(6R) and TPP1(4R) mutants. C, ubiquitination assays were performed as in B using TPP1–3xHA constructs containing Lys to Arg mutations or Lys residues only at the positions indicated. Lys-232 and Lys-233 of TPP1–3xHA are ubiquitinated. D, ubiquitination assays were performed as in B using TPP1 constructs containing Lys to Arg mutations or Lys residues only at the positions indicated. Any of the C-terminal four Lys residues of TPP1–3xHA can be ubiquitinated.