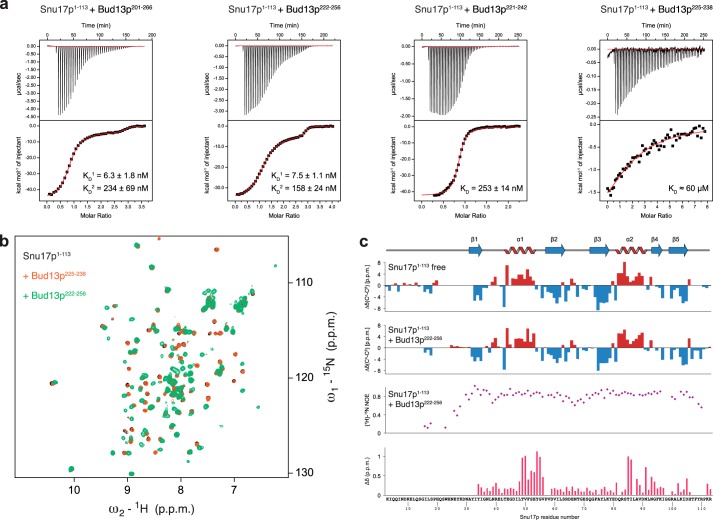
FIGURE 3.
ITC and NMR analysis of Snu17p binding to Bud13p. a, four different Bud13p peptides containing the invariant Trp residue were tested by ITC titrations for Snu17p binding. Binding curves for a 66-mer, a 35-mer used in the accompanying structural studies, a 22-mer, and a 14-mer Bud13p peptide, are depicted from left to right. Dissociation constants obtained by fitting the isotherms after subtracting the heat of dilution are listed. b, overlay of 1H,15N HSQC spectra of free Snu17p (residues 1–113, black) and after titration with the shortest Bud13p ligand (residues 225–238, orange) or with the optimal Bud13p peptide (residues 222–256, green). c, secondary 13C chemical shifts Δδ(13Cα)-Δδ(13Cβ) for Snu17p free and bound to Bud13p-(222–256), {1H}-15N heteronuclear NOE of the Snu17p-Bud13p complex, and chemical shift perturbations of Snu17p amide signals (recorded on a sample of 15N-labeled Snu17 residues 25–113) upon binding to Bud13p-(222–256) are plotted versus the amino acid sequence of Snu17p. Secondary structural elements of Snu17p are indicated on top.