FIGURE 1.
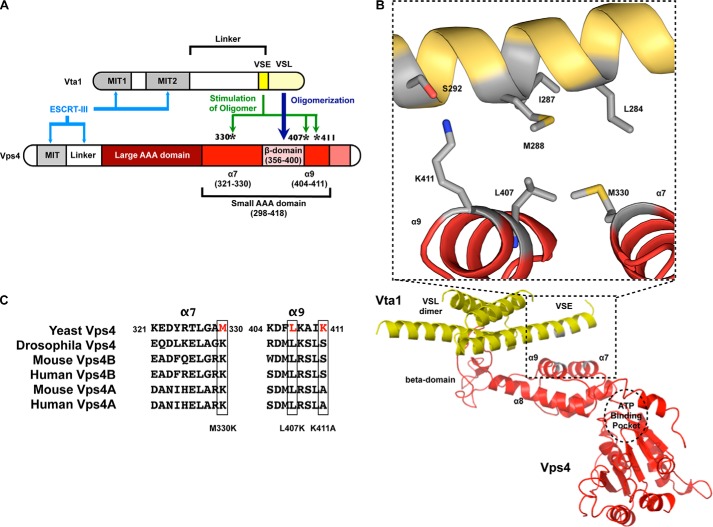
Modeling of the VSE into the structure of the VSL domain in complex with the small AAA and β-domains of Vps4. A, cartoon of Vps4 structural domains. The β-domain is an insert within the small AAA domain. Met-330 is in α7 preceding the β-domain, whereas Leu-407 and Lys-411 are in α9 immediately following the β-domain. α9 is located closer to the β-domain (proximal helix), whereas α7 is more distant (distal helix). B, the structure of the Vta1 VSL dimer bound to the Vps4 β-domain and small AAA domain (Protein Data Bank code 3MHV; MMDB 85335) was used to align structures of the Vps4 AAA domain (residues 119–437) (Protein Data Bank code 2QP9; MMDB 59522; red) and the Vta1 carboxyl terminus (residues 281–330) (Protein Data Bank code 2RKL; MMDB 62002; yellow). The Vta1 VSE falls on a flexible helix preceding the VSL domain, and alignment positioned the VSE neighboring Vps4 α7 and α9. Residues of the Vta1 VSE (Leu-284, Ile-287, and Met-288) and Vps4 small AAA domain (Met-330 of α7 and Leu-407 of α9) that appear to mediate VSE-Vps4 interaction are indicated in gray in the inset. Vta1 Ser-292 is not required for VSE stimulation but appears to interact with Vps4 Lys-411 of α9. These residues are also depicted in gray. C, sequence alignment of α7 and α9 from S. cerevisiae Vps4, D. melanogaster Vps4, M. musculus Vps4A and Vps4B, and H. sapiens Vps4A and Vps4B. Leu-407 is conserved from yeast to man.