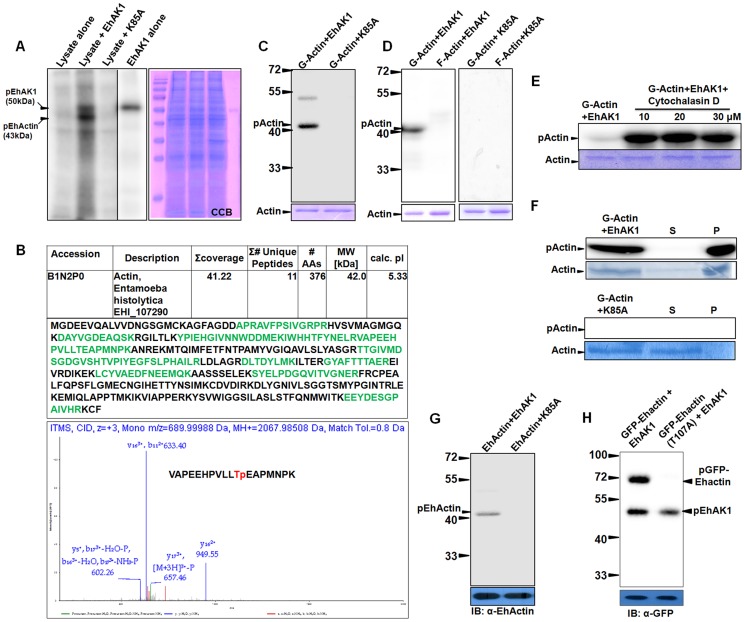
Figure 7. EhAK1 phosphorylates G-actin at threonine 107.
All phosphorylation reactions were carried out in vitro and products were resolved on SDS-PAGE. Radiolabeled products were visualized in a phosphorimager. (A) Phosphorylation of Ehactin by EhAK1. Total cell lysate (200µg) was incubated with γ-32P-ATP in the presence of EhAK1 (2µg). Reaction was stopped with SDS sample buffer containing EDTA after boiling and samples were resolved on 12% SDS-PAGE. Kinase dead mutant K85A was used as negative control. (B) Identification of phosphoylation site of Ehactin. The 43 kDa pEhactin band identified in panel (A) was cut out and subjected to mass spectrometry (LC-MS/MS). Table shows summary of the mass spectrometry results, and the peptides that mapped to Ehactin sequence. The CID-MS3 spectrum of the Ehactin VAPEEHPVLLTpEAPMNPK phosphopeptide showed 11th threonine position to be phosphorylated. Prominent y and b ions are shown, and [M + 3H]3+−P = 658. (C) Phosphorylation of rabbit skeletal muscle actin by EhAK1. Rabbit actin (2µg) was incubated with either EhAK1 (2µg) or mutant K85A-EhAK1 (2µg) in presence of kinase assay buffer. The input actin visualized by coomassie staining, is shown in the lower panel. (D) Phosphorylation of rabbit G-actin by EhAK1. G-actin and F-actin were separated by ultracentrifugation (1, 00,000 g) as supernatant (G-actin) and pellet (F-actin). The separated fractions (2µg) were used for kinase assay in the presence of EhAK1. (E) Phosphorylation of rabbit G-actin by EhAK1 in the presence of actin polymerization inhibitor cytochalasin D. (F) Polymerization of phosphorylated rabbit G-actin. G-actin (2µg) was phosphorylated in the presence of EhAK1 (2µg) or K85A (2µg) followed by ultracentrifugation at 1, 00,000 g to separate supernatant (G-actin) and pellet (F-actin). (G) Phosphorylation of Ehactin by EhAK1. E. histolytica actin was immunoprecipitated from the total lysate with anti Ehactin antibody. Immunoprecipitated material was used for setting up a kinase reaction with EhAK1. (H) Phosphorylation of GFP-tagged wild type and mutant Ehactin by EhAK1. Over-expressed GFP-tagged wild type or mutant Ehactin was immunoprecipitated from total lysate with anti-GFP antibody. Immunoprecipitated material was used for setting up a kinase reaction with EhAK1.