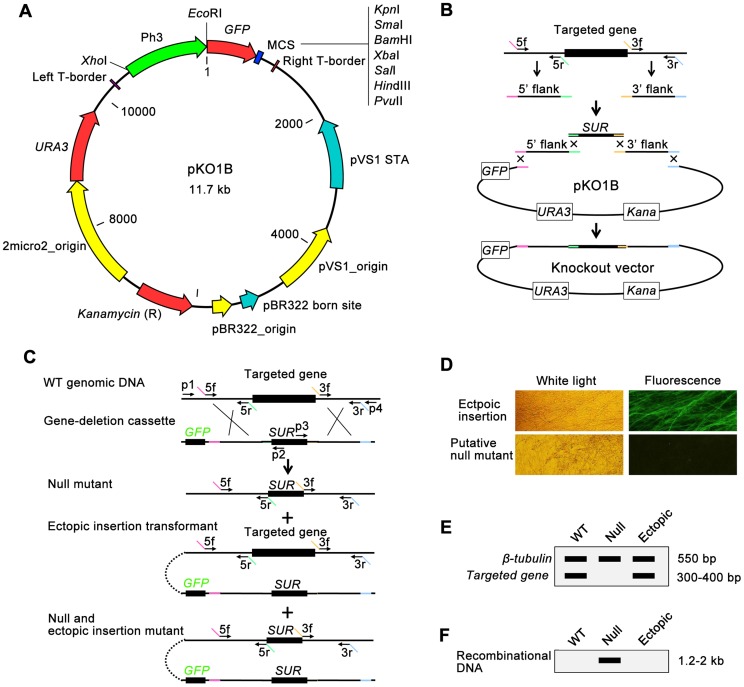
Figure 1. Overview of the high-throughput gene knockout system in fungus.
(A) Features of a new binary yeast-Escherichia-Agrobacterium shuttle vector, pKO1B. (B) Building of gene-deletion cassettes in pKO1B by yeast recombinational cloning. The 5′ and 3′ flanking fragments of the targeted genes were separately amplified from genomic DNA with primers 5f/5r and 3f/3r. Primers 5r and 3f have 5′ tails homologous to the SUR cassette, whereas those for 5f and 3r are homologous to the vector. The two flanks were cotransformed into yeast along with the SUR cassette and gapped pKO1B. Homologous recombination created the circular knockout vector, and the final knockout vector was subsequently transformed into A. tumefaciens. (C) Deletion of the targeted gene. The gene-deletion cassette was transformed into the fungal cells via ATMT. Homologous recombination created three types of transformants: null mutants, ectopic insertion transformants, and null and ectopic insertion mutants. The GFP gene was discarded in the null mutants. Primers p1/p2 or p3/p4 were used to identify the unique recombinational DNA fragment, indicating a knockout event. (D) The transformants are screened for GFP fluorescence under a microscope. Putative null mutants do have not GFP fluorescence, but ectopic transformants do. (E) The transformants are screened by double PCR for the targeted gene using the β-tubulin gene as a positive control. The wild-type strain or ectopic transformants produced a characteristic band, indicating the targeted gene, while the null mutants did not. (F) The transformants are screened by PCR for a unique recombinational DNA fragment marked as a knockout event. The null mutants have a 1.2–2.0 kb band on an electrophoretic gel, while the wild-type strain and the ectopic transformants do not.