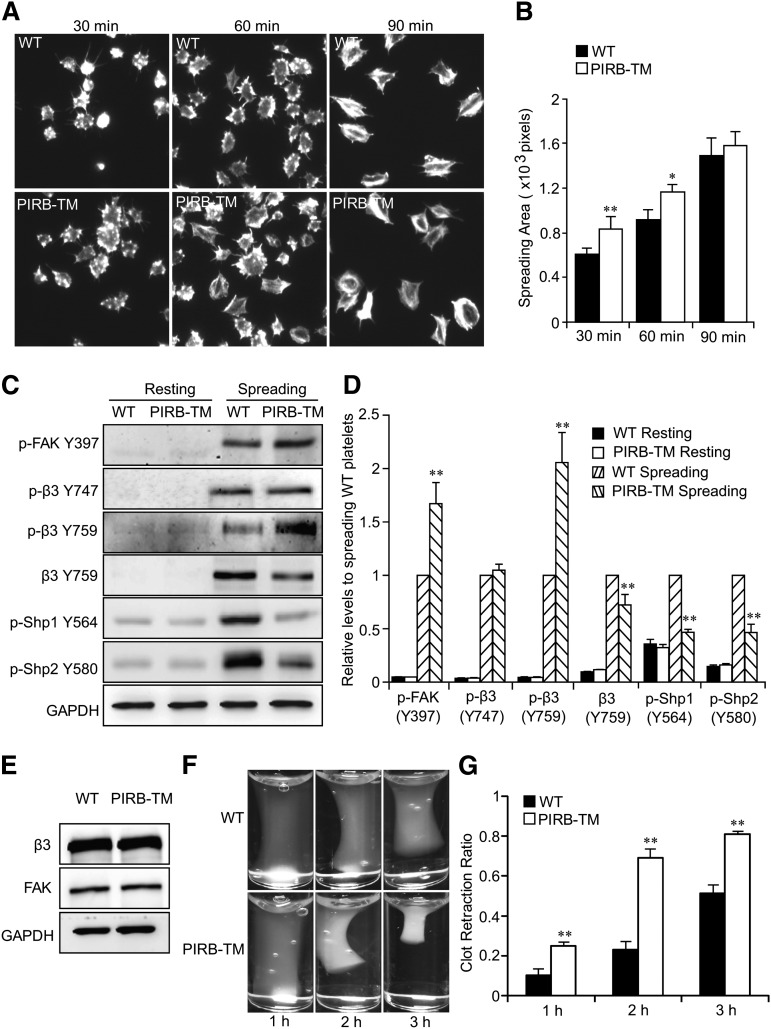
Figure 4.
PIRB regulates integrin αIIbβ3-mediated outside-in signaling. (A) Representative phalloidin-stained images of washed WT and PIRB-TM platelets spreading on immobilized Fg for 30, 60, and 90 minutes, respectively. (B) Quantification of the areas (pixel number) of 4 random fields per experiment, and ≥3 independent experiments were performed. Statistical analyses were performed using the Student t test (mean ± standard deviation [SD]; *P < .05; **P < .01). (C) Washed WT or PIRB-TM mouse platelets spread on 40 µg/mL Fg for 90 minutes. The adherent platelets were solubilized and analyzed by western blotting for detection of the phosphorylation of FAK Y397, integrin β3 Y747 and Y759, and Shp1 Y564 and Shp2 Y580 and cleavage of integrin β3 Y759. GAPDH was used to verify equal loading. (D) Densitometry measurements from results in C. Values were normalized with respect to WT spreading for each immunoblot and are expressed as relative levels to spreading WT platelets. Statistical significance was determined using the Student t test. (n = 3, mean ± SD; **P < .01). (E) The expression levels of β3 and FAK in WT and PIRB-TM platelets. GAPDH was used to verify equal loading. (F) Clot retraction of PRP containing WT and PIRB-TM platelets in the presence of 0.5 U/mL thrombin. (G) Two-dimensional retraction of clots was measured, and the data were expressed as retraction ratios. Statistical significance was calculated using the Student t test (n = 5, mean ± SD; **P < .01).