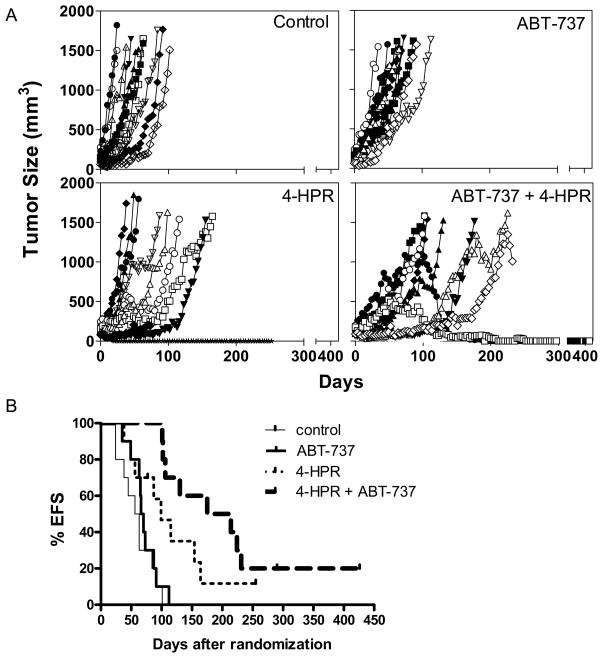
Figure 5. In vivo antitumor activity of ABT-737 combined with 4-HPR against human neuroblastoma cells.
Athymic (nu/nu) mice carrying CHLA-119 NB subcutaneous xenografts were treated with vehicle control (thin lines), ABT-737 (bold lines), 4-HPR (thin dotted lines) or 4-HPR + ABT-737 (bold dotted lines). 4-HPR/LYM-X-SORB oral powder in water (240 mg/kg) was given by oral gavage five days/week. ABT-737 (100 mg/kg) was administered by i.p. injection 5 days/week. When used in combination, ABT-737 was administered at a dose of 50 mg/kg/day of ABT-737 (half of the dose used in single agent treatment). (A) Tumor volumes were measured twice per week and tumor volumes calculated as described in materials and methods. Each line represents tumor growth in a mouse. Mice were sacrificed when tumors reached 1,500 mm3. (B) The event-free-survival (EFS) of mice was calculated from the time of tumor injection to the time the tumor volume reached 1,500 m3 or the mice had to be sacrificed due to treatment-related toxicity. Each line represents the proportion of mice remaining event-free over time.