Figure 3. Interrelations between the miRNA-bioprocessing machinery, stress and stress granules.
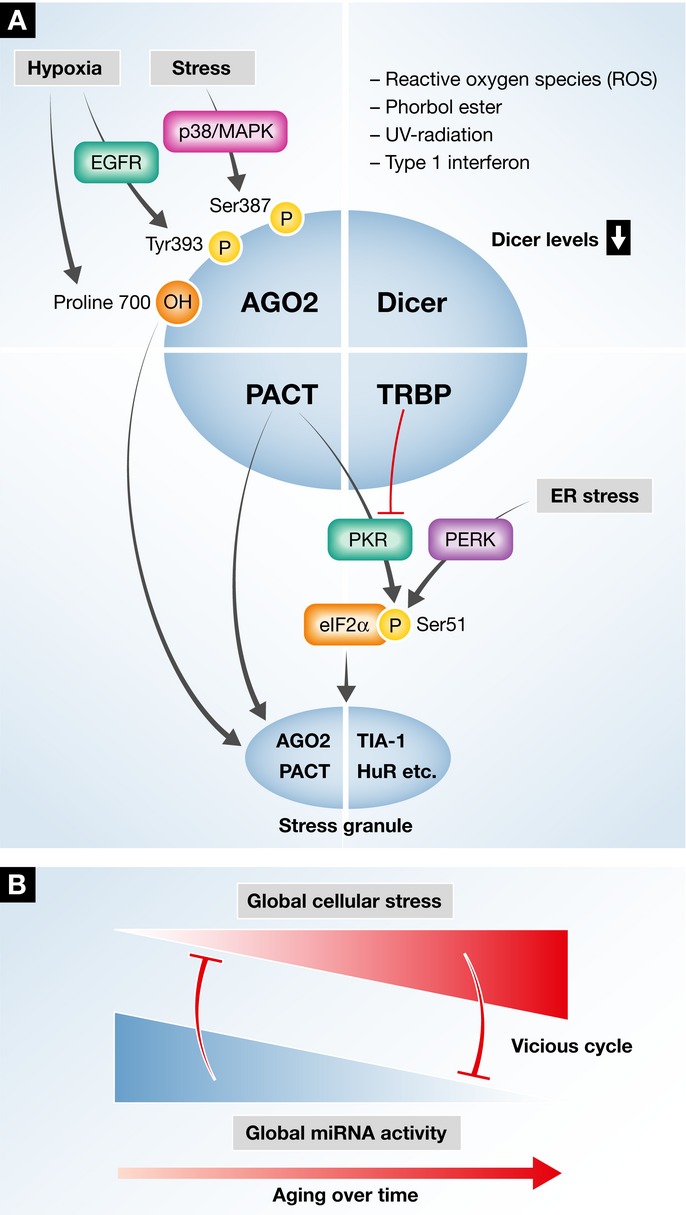
(A) A simplified illustration of the Dicing complex with its documented components: Dicer, AGO2, TRBP and PACT. Stress was suggested to modify AGO2 at a post-translational level: Hypoxia induces Proline 700 hydroxylation, which recruits AGO2 into stress granules. Likewise, EGFR-mediated phosphorylation of Tyrosine 393 leads to impaired miRNA processing of specific pre-miRNAs. Stress-mediated p38/MAPK signaling phosphorylates AGO2 on Serine 387, but the impact of this phosphorylation event on miRNA maturation is not characterized. Several types of cellular stress down-regulate Dicer levels and inhibit miRNA processing. Kinases of eukaryotic translation initiation factor 2 alpha (eIF2a) phosphorylate it on Serine 51, which is a critical step in halting translation and inducing stress granules. eIF2a is phosphorylated by eIF2aK3/PERK as part of the unfolded protein response, in response to endoplasmic reticulum stress (ER stress), or by eIF2aK2/PKR in response to viral doubled-stranded RNA. For simplicity, eIF2aK1 and eIF2aK4 are not mentioned. eIF2aK2/PKR activity is modulated by PACT and TRBP in a reciprocal manner. When stress granules are formed, both PACT and AGO2 are recruited into them. The interactions between cellular stress and the Dicing complex/miRNA-RISC-loading complex core components point toward a dynamic regulation of Dicing activity. (B) Stress signaling potentially alters the function of the miRNA-bioprocessing core components and leads to decompensated regulation. The consequences may include a vicious cycle, whereby stress impairs miRNA processing which in turn renders cells even more prone to stress.
