Figure 6. ATM-dependent phosphorylation of Rad17 at Thr622 is required for HR repair.
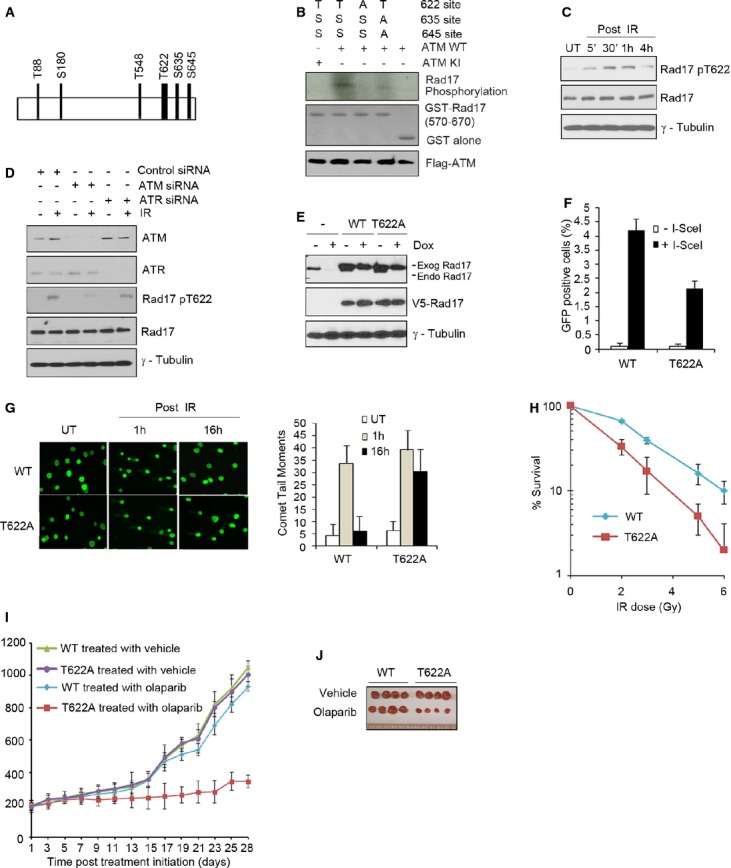
A Schematic presentation of potential phosphorylation TQ/SQ motifs in Rad17.
B ATM directly phosphorylates Rad17 at Thr622. An in vitro ATM kinase assay was performed as described in Supplementary Material and Methods.
C U2OS cells were either untreated or exposed to 5 Gy of IR. Western blot was performed as indicated.
D ATM but not ATR is responsible for Rad17 phosphorylation at Thr622 following DSB damage. U2OS cells transfected with the indicated siRNA were either untreated or exposed to 5 Gy of IR. Cell lysates were prepared 1 h after IR. Western blot was performed.
E Endogenous Rad17 is depleted by Dox induction and replaced by exogenous Rad17. Inducible Rad17 knockdown cells (U2OS) stably expressing shRNA-resistant Rad17 WT or Rad17 T622A mutant were treated with or without Dox for 2 days. Western blot was performed.
F–H Rad17 Thr622 phosphorylation is required for HR repair and cell survival. Inducible Rad17-knockdown cells reconstituted with shRNA-resistant Rad17 WT or Rad17 T622A mutant were used. HR repair assay was performed as previously described (F); comet assay was performed as previously described (G); clonogenic survival assay was performed using IR treatment (H).
I, J Rad17 Thr622 phosphorylation is necessary for tumor growth in the presence of PARP inhibitor. Mice bearing xenograft tumors of 200–220 mm3 were divided into two groups for each cell line as indicated: vehicle control group and Olaparib group. Each group contained four nude mice. Tumor size was measured and tumor growth curves plotted (I); tumors were harvested on day 28 after the start of Olaparib treatment (J).
Data information: Error bars represent mean ± s.d. (n = 4).
Source data are available online for this figure.
