Figure 7. Thr622 phosphorylation is required for Rad17-NBS1 interaction.
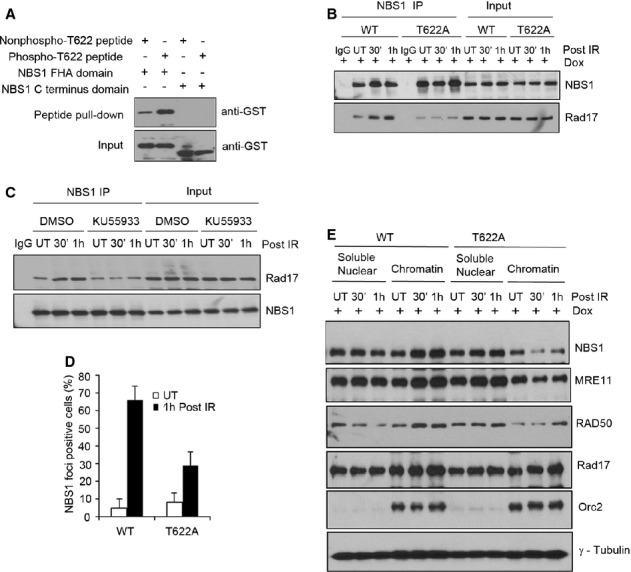
A Rad17 Thr622 phosphorylation is required for the interaction between Rad17 and the FHA domain of NBS1. Streptavidin-Sepharose beads coupled with the indicated peptides were incubated with purified GST-NBS1 fragments (FHA domain or C-terminus domain containing residues 682–754). Western blot was performed using anti-GST.
B, D–E The mutation of Thr622 phosphorylation attenuated the Rad17-NBS1 interaction and the recruitment of NBS1 to damaged chromatin. Inducible Rad17 knockdown cells (U2OS) stably expressing shRNA-resistant Rad17 WT or Rad17 T622A mutant treated with Dox for 2 days were either untreated or exposed to 5 Gy of IR. Cell lysates were subjected to immunoprecipitation with anti-NBS1 as indicated (B); Immunostaining was performed, and the percentage of NBS1 foci-positive cells was plotted (mean ± s.d., n = 3) (D); soluble nuclear and chromatin fractions were isolated and Western blot was performed (E).
C Inhibition of ATM kinase activity reduced the interaction of Rad17 with NBS1. U2OS cells were either untreated or treated with KU55933 (ATM inhibitor, 10 μM) for 2 h. IP experiment was performed as indicated.
Source data are available online for this figure.
