Summary
Background
C-reactive protein (CRP) promotes tissue factor (TF) and plasminogen activator inhibitor-1 (PAI-1) expression in vitro, and elevated plasma CRP concentration is associated with increased risk of vein graft (VG) thrombosis after coronary artery bypass surgery. However, little is known about the effects of CRP on VG TF and PAI-1 expression in vivo, or on VG thrombosis.
Objectives
We studied transgenic (Tg) mice expressing human CRP in a VG model to explore in vivo cause-and-effect relationships between CRP and TF, PAI-1, and VG thrombosis.
Methods
Vein segments from WT and CRP-Tg donors were transplanted into carotid arteries of WT and CRP-Tg recipients. VGs were analyzed 1-4 weeks later.
Results
Human CRP accumulated in VGs during the first 4 weeks after surgery, but appeared to originate exclusively from systemic sources, rather than local production. Human CRP significantly increased TF gene expression, protein concentration, and activity in VGs. Human CRP also increased PAI-1 concentration in VGs, though only in vascular endothelial cells. Human CRP stimulated macrophage migration, invasion into VGs, and TF expression. Fibrin deposition was significantly greater in VGs of CRP-Tg mice compared to WT controls.
Conclusions
CRP accumulates in VGs early after surgery, originating from systemic sources rather than local synthesis. Human CRP promotes TF and PAI-1 expression in VGs, though with different expression patterns. Human CRP stimulates macrophage invasion and fibrin deposition within VGs. These results suggest that CRP induces pathological changes in VGs that contribute to early VG occlusion.
Keywords: C-reactive protein, plasminogen activator inhibitor-1, thrombosis, tissue factor, vascular graft occlusion
Saphenous veins are used to perform coronary artery bypass graft (CABG) surgery in patients with advanced obstructive coronary artery disease. The procedure involves connecting one end of the harvested saphenous vein segment to the aorta and the other end to a coronary artery, distal to its obstructed segment. Early vein graft (VG) thrombosis, defined as that occurring within 30 days after surgery, is an important clinical problem that occurs in 3-12% of patients. Multiple factors are involved, including traumatic injury of the VG during surgery, endothelial cell denudation and dysfunction, turbulent or diminished blood flow, and inflammation [1]. However, the pathogenesis of VG thrombosis is inadequately understood, which has limited strategies to prevent this important clinical problem.
C-reactive protein (CRP) is an acute-phase reactant plasma protein that plays a key role in innate immune responses [2]. In humans, CRP expression increases markedly in response to inflammatory stimuli, such as surgery [3]. Elevated plasma CRP is associated with increased risk of early VG occlusion [4, 5]. In addition, CRP is present in the wall of VGs, where it may exert important biological effects [6, 7]. In vitro, CRP induces tissue factor (TF) expression by vascular endothelial cells and smooth muscle cells (SMCs) [8-10], and plasminogen activator inhibitor-1 (PAI-1) by endothelial cells [11, 12]. These effects would be expected to promote thrombosis. However, the impact of enhanced CRP expression on TF and PAI-1 expression in VGs in vivo remains undefined, and it is unknown whether elevated CRP expression promotes VG thrombosis or is simply a biomarker associated with increased thrombotic risk.
The goals of this study were to examine the effects of CRP on TF and PAI-1 expression in VGs and to determine if increased CRP expression promotes fibrin formation and thrombosis in VGs. To achieve our objectives we utilized a VG surgery model and human CRP-transgenic (Tg) mice bearing the full-length human CRP gene, including its natural promoter [13]. Our results suggest that CRP is a driver of pathological processes that contribute to early VG thrombosis.
Materials and Methods
Reagents
Purified human recombinant CRP (Trichem Resources) was dialyzed extensively to remove sodium azide and passed over Detoxigel (Pierce). Bacterial endotoxin concentration in CRP preparations was <0.06 endotoxin units/mL.
Mice
C57BL/6J mice were from Jackson Laboratories. C57BL/6-congenic CRP-Tg mice were described previously [13]. Males were used because male CRP-Tg mice have elevated baseline expression of human CRP that responds in acute-phase pattern to inflammatory stimuli [14] and male blood vessels are significantly larger than those of females, facilitating VG surgery [14, 15]. Mice received normal chow (5008/LabDiet). Animal care and experimental procedures were approved by the University of Missouri Animal Care and Use Committee. Surgery and histological analyses were performed with the investigators blinded to mouse genotypes.
Vein grafting surgery
A segment of inferior vena cava (IVC) from a donor mouse was surgically implanted into the right common carotid artery of a recipient mouse, as described [15].
Measurement of plasma CRP and PAI-1
Human CRP concentration was measured with a human CRP ELISA kit (Alpco). PAI-1 concentration was measured using the mouse PAI-1 total antigen ELISA kit (Molecular Innovations).
Immunohistochemistry
VGs were processed and immunostained as described [16]. Negative controls omitting primary antibody or substituting a non-immune, isotype control primary antibody were performed to confirm specificity of primary antibodies (Supplemental Fig. 1, online supplement). CRP and PAI-1 were detected using rabbit anti-human CRP polyclonal antibody and rabbit anti-mouse PAI-1 polyclonal antibody (Santa Cruz Biotechnology), respectively, followed by peroxidase-conjugated anti-rabbit-IgG. TF was detected using rabbit anti-mouse TF polyclonal antibody (American Diagnostica) and DyLight488 goat anti-rabbit IgG. Reactions performed with a second rabbit anti-mouse TF polyclonal antibody (abcam) yielded similar results (Supplemental Fig. 1, online supplement). Macrophages were detected with rat anti-Mac-3 IgG (BD Pharmingen) and Texas-Red-conjugated goat-anti-rat IgG antibody. Fibrin was detected with anti-fibrin antibody that does not cross-react with fibrinogen (Bβ 15-42, clone T2G1; Accurate Chemical); secondary antibody was Dylight 594 horse anti-mouse antibody. Endothelial cells were immunostained with rabbit anti-mouse CD31 IgG (abcam). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). To quantify CRP, TF, PAI-1, and macrophage immunostaining, images of cross-sections were captured under identical conditions and imported into Image-Pro Plus software. Four regions of interest (ROI) were drawn within neointima at the 12, 3, 6, and 9 o'clock positions or directly overlying the vascular endothelium. Amount of positive immunostaining within each ROI (expressed as positive pixels/mm2) was determined by false color segmentation analysis and averaged. To quantify fibrin immunostaining the entire circumference of the VG wall (rather than 4 ROIs) was analyzed.
Real-time reverse transcriptase (RT) PCR
Total cellular RNA was isolated from veins and livers using RNeasy Micro kit (Qiagen) and subjected to real-time RT-PCR using the SYBR Green method (BIO-RAD). PCR primers were 5′CAATATACCCAGGCCACAAG3′ and 5′AATCCAGCAGCAAGAAGATG3′ (human CRP), 5′-GCTGCAGATGACCACAGCGGG-3′ and 5′-CCGCAGTACTGATCTCATTC-3′ (murine PAI-1), 5′GCTATGATTTTCTCCAGGAAAA3′ and 5′GTCCCGCTCGGTTCTTTCTGCGC3′ (murine TF), and 5′AAGAGCTATGAGCTGCCTGA3′ and 5′TACGGATGTCAACGTCACAC3′ (β-actin). Gene expression was quantified as described [17].
Macrophage migration assay
Murine macrophages were harvested from the peritoneal cavity 4 days after intraperitoneal injection of thioglycollate. Macrophage migration was studied in Transwell inserts (Corning) containing porous membrane bottoms (8 μm pore diameter). Macrophages (3×105, suspended in RPMI-1640 containing 10% fetal bovine serum [FBS]) were placed in the upper chamber and RPMI1640/10% FBS containing monocyte chemoattractant protein-1 (MCP-1, 10 ng/mL, R&D Systems) and CRP (0-40 μg/mL) were placed in the lower chamber. After 24 hours, the number of cells that migrated to the lower chamber was counted.
Measurement of TF activity in vein grafts and macrophages
VGs were excised, rinsed, frozen in liquid nitrogen, ground to a powder while cooled, suspended in Tris-HCl (50 mM, pH 8.0), subjected to 3 freeze/thaw cycles, and centrifuged. Supernatant total protein concentration was adjusted to 400 μg/mL. Peritoneal macrophages (106) were added to 96-well microtiter plates in RPMI-1640 medium. Purified human CRP (0-50 μg/mL) was added. After 6 hours at 37°C cells were harvested and lysed in RIPA buffer. TF activities of VG and macrophage lysates were measured by adding samples (10-25 μL) to reaction mixtures containing human factor VIIa (3 nM, Haematologic Technologies), human factor X (100 nM, Haematologic Technologies), 8.3 mM CaCl2, and Spectrozyme fXa (0.33 mM, American Diagnostica). After 30-45 min absorbances of reaction mixtures at 405 nm (A405) were measured. TF activity was determined from a standard curve of recombinant human TF (American Diagnostica) or expressed as ΔA405/min. Chromogenic substrate hydrolysis was negligible if factor VIIa or X were omitted from reactions.
Mechanical VG injury model
Seven days after VG surgery mice were anesthetized. VGs were surgically exposed and subjected to focal mechanical injury, as described [18], with minor modification. In brief, three 6-0 silk sutures were tied in knots around the VG to produce a focal crush injury ∼2 mm in length. After 3 minutes knots were removed, restoring vascular flow. A probe (Transonic Systems) was placed on the VG to monitor blood flow. After 60 minutes the VG was excised, washed 3 times, blotted dry, frozen in liquid nitrogen, pulverized, incubated 30 minutes in RIPA buffer (THERMO Scientific) containing protease inhibitors, and centrifuged. A portion of each supernatant (total protein 15 μg) was subjected to reducing SDS-PAGE and Western blotting. Primary antibody was Bβ 15-42 anti-fibrin antibody; secondary antibody was horse-radish-peroxidase-conjugated goat anti-mouse IgG H+L (Jackson ImmunoResearch) [18]. NIH Image J software was used to quantify fibrin band intensities. Control experiments showed that fibrin accumulation in VGs 7 days after surgery was barely appreciable in the absence of mechanical injury and markedly enhanced 60 minutes after mechanical injury.
Ferric chloride VG injury model
Induction of thrombosis and monitoring of VG blood flow were performed as described previously [19], except that 2 pieces of 1M Whatman filter paper (1×2 mm), each saturated with 10% FeCl3 solution, were placed on opposite sides of the VG for 3 minutes. Time to occlusion was measured.
Statistical Analyses
Results are expressed as mean ± standard error of the mean. One way analysis of variance with pairwise multiple comparisons, Student's t test, log-rank test, and Mann-Whitney rank sum test were used, as appropriate.
Results
Human CRP transgene is not expressed in VGs
To determine if human CRP is expressed in IVCs of CRP-Tg mice, total cellular RNA was prepared from IVC segments of WT and CRP-Tg mice and subjected to RT-PCR using primers specific for human CRP. Compared to positive control (i.e. CRP-Tg liver), where the transgene is known to be expressed at high levels in healthy male mice [13, 14], transgene expression in CRP-Tg IVC segments was essentially undetectable and indistinguishable from negative controls (i.e. WT IVCs and livers; Fig. 1A). To determine if human CRP transgene expression is up-regulated after surgery, we transplanted a segment of WT IVC into WT recipient mice (designated WTdonor/WTrecipient mice), CRP-Tg IVC into WT recipient mice (CRP-Tgdonor/WTrecipient mice), and CRP-Tg IVC into CRP-Tg recipient mice (CRP-Tgdonor/CRP-Tgrecipient mice). RT-PCR analysis of livers harvested from CRP-Tgdonor/CRP-Tgrecipient mice 7 days later revealed that transgene expression was increased greater than two-fold compared to livers of donor CRP-Tg mice (Fig. 1B). However, human CRP transgene expression was essentially undetectable in VGs harvested from CRP-Tgdonor/WTrecipient mice and CRP-Tgdonor/CRP-Tgrecipient mice, with both groups being indistinguishable from negative controls (i.e. VGs harvested from WTdonor/WTrecipient mice; Fig. 1B). By 28 days after surgery human CRP transgene expression in CRP-Tgdonor/CRP-Tgrecipient mice returned to baseline in livers and remained undetectable in VGs (Fig. 1C).
Figure 1.
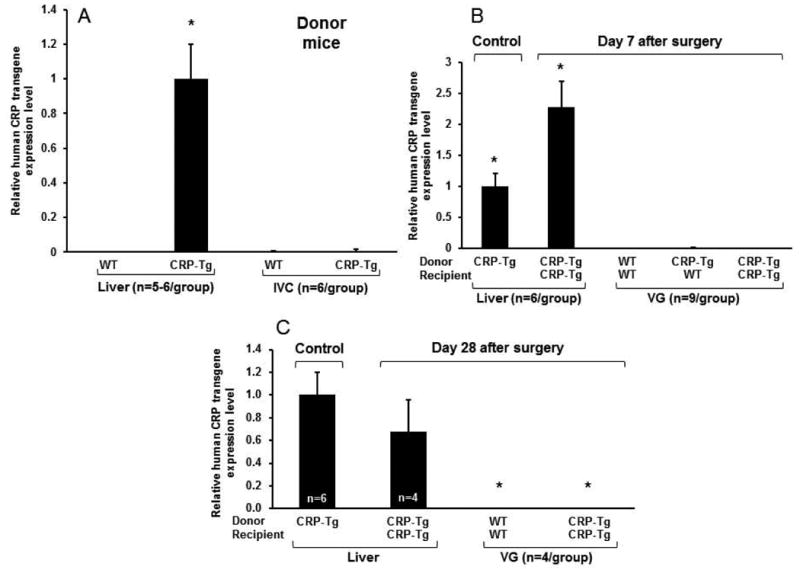
Quantitative RT-PCR analysis of human CRP transgene expression in mouse livers, inferior vena cavae (IVC), and vein grafts (VGs). (A) Human CRP is expressed in liver, but not IVCs of CRP-Tg donor mice. CRP transgene expression level in liver was sufficient to produce high baseline high levels of human CRP in CRP-Tg mouse plasma (see Fig. 3) and was used as the positive control (assigned expression level 1.0). *P<0.001 vs. all other groups. (B) At 7 days after surgery human CRP transgene expression in livers is significantly increased above donor liver control, but is negligible in VGs. *P<0.001 vs. all other groups. (C) At 28 days after surgery expression of human CRP transgene in livers returns to a level that does not differ significantly from donor liver control (P>0.1) and remains negligible in VGs (*P<0.005 vs. donor liver control).
Human CRP protein is present in VGs
At 7 days after surgery we observed a significant increase in human CRP immunostaining in VGs harvested from CRP-Tgdonor/CRP-Tgrecipient mice compared to WTdonor/WTrecipient mice (Fig. 2 A and B). At 28 days after surgery we found very weak or undetectable human CRP immunostaining in VGs from CRP-Tgdonor/WTrecipient mice, which did not differ significantly from negative controls (i.e. VGs from WTdonor/WTrecipient mice; Fig. 2 C and D). In contrast, strong human CRP immunostaining was observed in VGs from WTdonor/CRP-Tgrecipient mice and CRP-Tgdonor/CRP-Tgrecipient mice, with no significant difference in staining intensity between these two groups. Together, these findings suggested that CRP present in VGs originates from a systemic source, rather than local synthesis.
Figure 2.
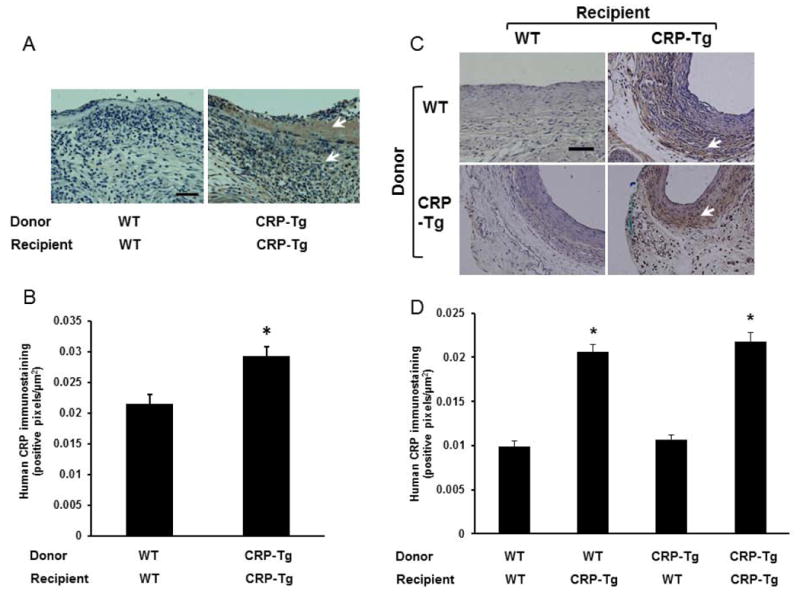
Human CRP immunostaining in VGs. (A) VGs harvested 7 days after surgery. Donor/recipient genotypes are as shown. (B) Quantitative analysis of intimal CRP immunostaining at 7 days after surgery (n=4 mice/group). *P<0.02 vs. WTdonor/WTrecipient group. (C) VGs harvested 28 days after surgery. For this time point all 4 potential combinations of donors and recipients were included. (D) Quantitative analysis of intimal CRP immunostaining at 28 days after surgery (n=3 mice/group). *P<0.01 vs. WTdonor/WTrecipient group. In panels (A) and (C) brown color (arrow heads) represents CRP immunostaining; scale bars = 50 μm.
Concentration of human CRP in mouse plasma is high and increases transiently after surgery
Human CRP concentration in plasma of CRP-Tg mice was high at baseline, rose significantly at 7 days after surgery, and returned to baseline by day 28, regardless of VG donor genotype (Fig. 3). Human CRP was undetectable in plasma of WT recipient mice, both at 7 and 28 days, including in WT mice that received CRP-Tg VGs.
Figure 3.
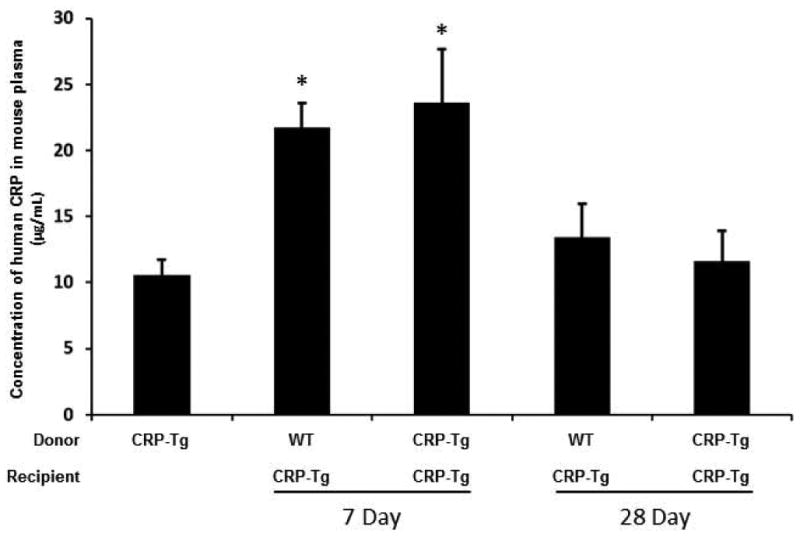
Concentration of human CRP in mouse plasma increases transiently after surgery and is not affected by VG genotype. Donor/recipient genotypes are as shown. CRP-Tg mice not subjected to surgery served as controls (1st bar). *P<0.02 vs. control; n=5-6 per group.
CRP stimulates TF expression in VGs
At 7 days after surgery, TF gene expression was significantly increased in VGs from CRP-Tgdonor/CRP-Tgrecipient mice compared to VGs from WTdonor/WTrecipient mice (Fig. 4A). There was an associated increase in VG TF protein concentration in CRP-Tg mice compared to WT controls, but the differences between groups did not achieve statistical significance (Fig. 4B). TF activity in protein extracts prepared from VGs harvested 7 days after surgery was significantly greater in CRP-Tg mice compared to WT controls (Fig. 4C). At 28 days TF gene expression was higher in VGs of CRP-Tg mice compared to WT mice, but the differences between groups did not achieve statistical significance (Fig. 4D). However, TF protein concentration in VGs of CRP-Tg mice was significantly greater than those of WT mice (Fig. 4E). As a whole, there results suggested that CRP accumulation in VGs stimulates TF expression.
Figure 4.
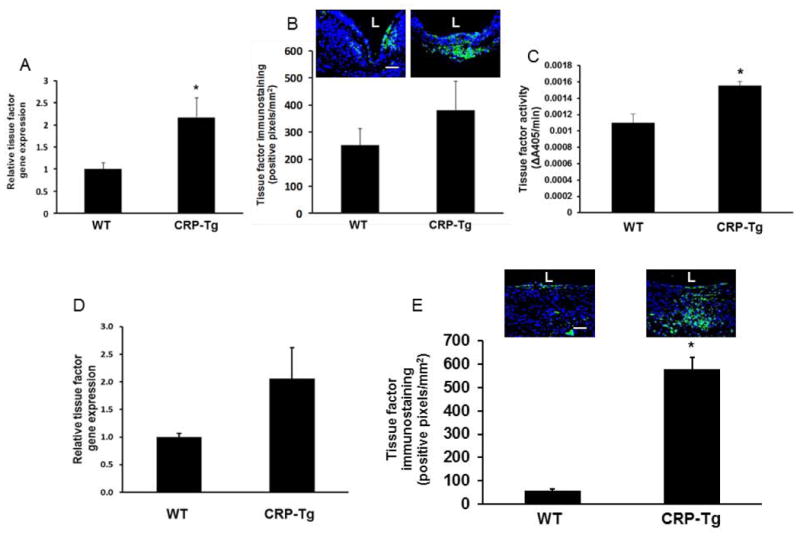
Effect of human CRP on TF expression, accumulation, and activity in VGs. (A) RT-PCR analysis of TF gene expression in VGs harvested 7 days after surgery. *P<0.05 vs. WT mice; n=6/group. (B) Immunofluorescence analysis of TF protein in VGs 7 days after surgery. Representative images of one VG from each group are shown; the difference between groups did not achieve statistical significance (P=0.19, n=4/group). (C) Factor VIIa cofactor activity in CRP-Tg VGs harvested 7 days after surgery was significantly greater than that of WT controls (*P<0.05, n=3/group). (D) RT-PCR analysis of TF gene expression in VGs 28 days after surgery. The difference between groups did not achieve statistical significance (P=0.2; n=4/group). (E) Immunofluorescence analysis of TF protein in VGs 28 days after surgery. Representative images and group data are shown. *P<0.01 vs. WT mice; n=5/group. In panels (B) and (E) green fluorescence represents TF, nuclei are stained blue, scale bars = 50 μm, and “L” denotes lumen of VG.
Effects of human CRP on PAI-1 expression in vivo
At 7 days after surgery we did not detect any significant difference in VG PAI-1 gene expression between WTdonor/WTrecipient mice and CRP-Tgdonor/CRP-Tgrecipient mice (Fig. 5A). However, PAI-1 protein concentration in the neointima and plasma PAI-1 concentration were significantly greater in CRP-Tg mice compared to WT controls (Fig. 5 B and C). Anti-CD31 immunostaining revealed that, regardless of genotype, vascular endothelial cells were largely absent from VGs at 7 days, whereas at 28 days the endothelium had essentially completely repopulated (Supplemental Fig. 2, online supplement). At 28 days, quantitative immunohistochemical analysis revealed that PAI-1 concentration in VG endothelium was significantly greater in CRP-Tg mice than in WT mice (Fig. 5D). However, PAI-1 concentration in the subendothelial VG neointima did not differ between groups (Fig. 5D), nor did plasma PAI-1 concentration (Fig. 5C). RT-PCR analysis revealed that there was a modest, statistically significant decrease in PAI-1 gene expression in VGs from CRP-Tg mice compared to VGs from WT controls (Fig. 5A).
Figure 5.
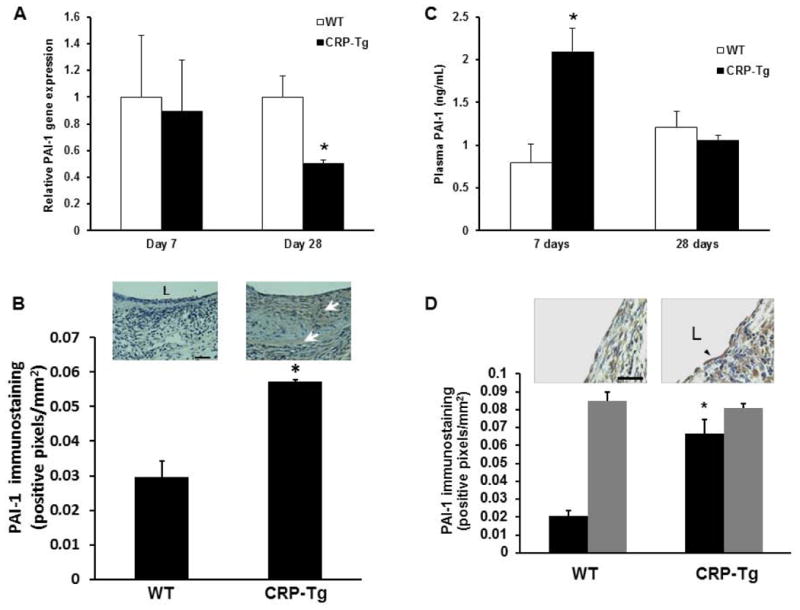
Effects of human CRP on PAI-1 expression. (A) Human CRP does not increase total PAI-1 gene expression in VGs (on Day 7, P>0.8; n=6/group; on Day 28, PAI-1 gene expression is significantly less in VGs of CRP-Tg mice than WT controls, *P<0.05, n=4/group). (B) Immunohistochemical analysis of PAI-1 protein in VGs 7 days after surgery. PAI-1 immunostaining (brown color, arrows) is significantly greater in VGs of CRP-Tg mice compared to VGs of WT mice (*P<0.02, n=4/group). Representative images are shown. Distance bar = 50 μm. “L” denotes lumen. (C) Plasma PAI-1 concentration is higher in CRP-Tg mice than in WT mice on day 7 after surgery, but not on day 28. *P<0.01 vs. WT mice; n=6/group at 7 days; n=4/group at 28 days. (D) On day 28 after surgery PAI-1 immunostaining is significantly greater in endothelial cells (black bars) of CRP-Tg VGs compared to WT controls (*P<0.01 vs. WT mice, n=4/group), whereas PAI-1 immunostaining within the subendothelial intima (gray bars) does not differ significantly between groups. Representative images are shown. Arrow head points to endothelial cells. Scale bar = 25 μm.
Human CRP increases macrophage migration, tissue factor expression, and accumulation in VGs
CRP binds to blood monocytes [20], which triggers inflammatory pathways linked to thrombosis [21]. Furthermore, macrophages invade into the wall of VGs early during the remodeling process [22, 23]. We studied the effect of purified human CRP on macrophage migration in vitro and observed a dose-dependent enhancing effect (Fig. 6A). CRP also increased macrophage TF activity (Fig. 6B). We also quantified macrophage invasion into VGs. At 7 days the greater macrophage immunostaining in VGs of CRP-Tgdonor/CRP-Tgrecipient mice compared to VGs of WTdonor/WTrecipient mice was statistically insignificant (Fig. 6C). At 28 days the difference between groups was greater and achieved statistical significance (Fig. 6D).
Figure 6.
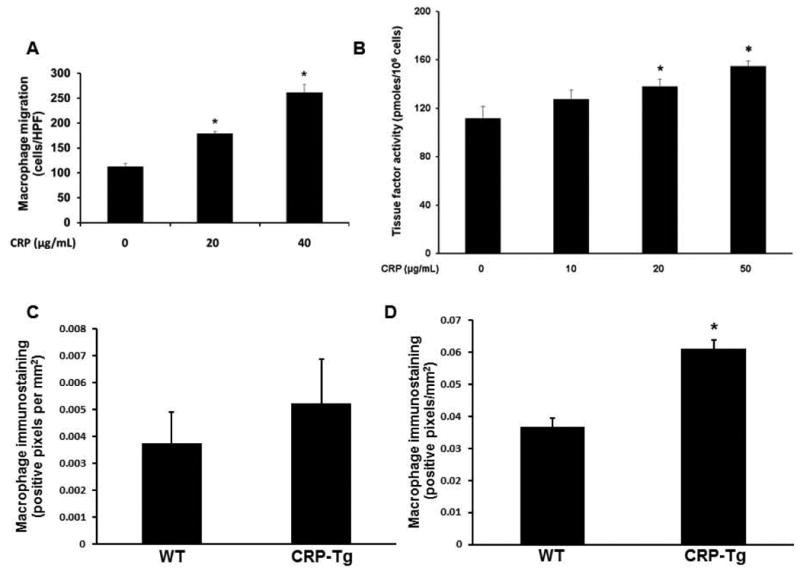
Human CRP increases macrophage migration, tissue factor (TF) activity, and invasion into VGs. (A) CRP increases migration of wild-type (WT) macrophages. Data are mean of 3 experiments *P<0.01 vs. other groups. (B) CRP increases TF activity associated with WT macrophages. Data are mean of 3 experiments. *P<0.05 vs. no added CRP control group. (C) Immunohistochemical quantification of macrophages in VGs 7 days after surgery; the difference between CRP-Tg and WT groups did not achieve statistical significance (P>0.4; n=4/group). (D) At 28 days after surgery macrophage content of VGs is significantly greater in CRP-Tg mice than in WT controls (*P<0.05; n=3 mice/group).
Effect of human CRP on fibrin formation and thrombosis in VGs
To study the effect of human CRP on activation of the blood coagulation system in VGs we performed surgery to generate WTdonor/WTrecipient mice (n=6) and CRP-Tgdonor/CRP-Tgrecipient mice (n=6). After 7 days VGs were re-exposed and subjected to 3 min of focal mechanical injury. This form of injury does not produce an occlusive thrombus, but allows quantitative assessment of mural fibrin formation [18]. Mechanical injury produced significantly greater fibrin formation in VGs of CRP-Tg mice compared to WT controls (Fig. 7A). We also used immunohistochemistry to study spontaneous fibrin formation within the wall of VGs, as this process has been described later during VG remodeling [22]. At 28 days after surgery intramural fibrin deposition was significantly greater in VGs from CRP-Tgdonor/CRP-Tgrecipient mice compared to VGs from WTdonor/WTrecipient mice (Fig. 7B). To study the effect of human CRP on formation of occlusive, platelet-rich thrombi in VGs, we generated WTdonor/WTrecipient mice (n=5) and CRP-Tgdonor/CRP-Tgrecipient mice (n=5), re-exposed VGs 7 days later, and subjected them to ferric chloride injury. Median occlusion time of the CRP-Tg group was 11 minutes compared to 13 minutes for WT mice (P=0.2, Fig. 8).
Figure 7.
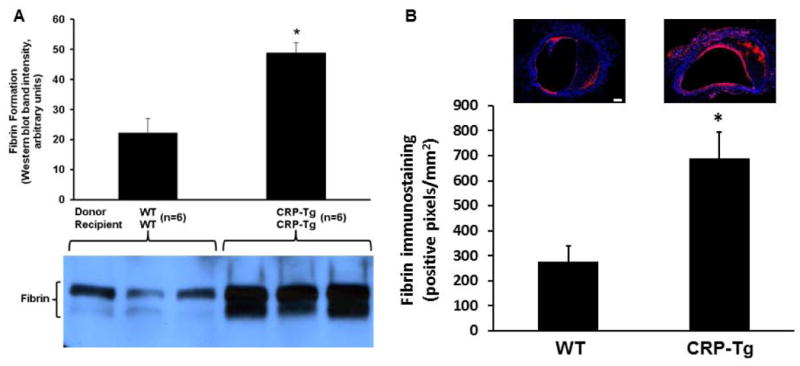
Human CRP enhances fibrin formation in VGs. (A) At 7 days after surgery human CRP significantly increases fibrin formation in mechanically injured VGs (*P<0.001 vs. WTdonor/WTrecipient mice; n=6 mice/group). Below the graph are shown raw fibrin immunostaining data for VGs obtained from 6 mice, 3 in each experimental group, as shown. (B) Spontaneous fibrin deposition is greater in CRP-Tg VGs than in WT VGs. VGs were harvested from WTdonor/WTrecipient mice and CRP-Tgdonor/CRP-Tgrecipient mice (n=6/group) at 28 days after surgery and subjected to fibrin-specific immunostaining. *P<0.02 vs. WT group. Representative images are shown. Red color represents fibrin. Nuclear (DAPI) staining is blue. Scale bar = 200 μm.
Figure 8.
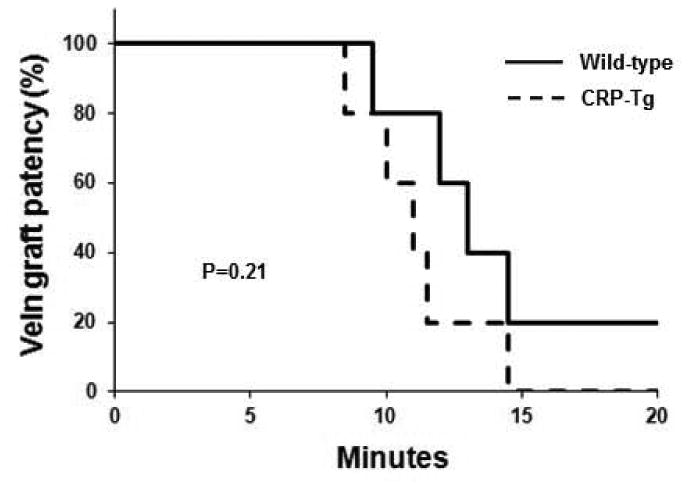
Effect of human CRP on ferric chloride-induced thrombosis. At 7 days after surgery the time course of formation of occlusive thrombi after ferric chloride injury does not differ significantly between CRP-Tgdonor/CRP-Tgrecipient mice and WTdonor/WTrecipient mice (n=5 mice/group).
Discussion
Two studies found a positive association between increased plasma CRP and early VG occlusion [4, 5], and in vitro mechanistic studies have identified potential pathways by which CRP might promote thrombosis. However, there has been a lack of data regarding the effects of CRP on prothrombotic pathways in VGs in vivo, and the potential existence of a cause-and-effect relationship between increased CRP expression and VG thrombosis has not been examined previously. To address this gap in knowledge we subjected male human CRP-Tg mice to VG surgery. In contrast to wild-type (WT) mice, in which endogenous CRP expression is very low at baseline and does not change appreciably in response to inflammation, liver expression of human CRP in male CRP-Tg mice is elevated at baseline and responds strongly to inflammatory stimuli [3, 14]. Therefore, our model enabled us to study the physiological impact of enhanced human CRP gene expression on VG thrombosis without the confounding variable of concomitantly enhanced murine CRP. In our study we documented only a modest, ∼2-fold increase in plasma human CRP after surgery compared to baseline values, most likely because our first blood sampling at 7 days after surgery was after the anticipated acute-phase peak in plasma human CRP, and because human plasma CRP is high at baseline in male CRP-Tg mice [14], which lowers the post-operative/pre-operative ratio. Nevertheless, our experiments allowed us to examine the impact of endogenously expressed human CRP on VG expression of PAI-1 and TF, two key pro-thrombotic factors, as well as on the thrombotic response of VGs to injury, both under conditions in which plasma concentrations of human CRP were consistent with those found in human diseases. A potential limitation of our transgenic model is that the functional binding interactions of human CRP with cells and other proteins may not be fully preserved in mice. However, several studies have shown that human CRP binds mouse Fc receptors, can activate murine complement, and exerts vascular effects via these pathways in CRP-Tg mice [24-26].
Our data suggest that human CRP deposits in the wall of VGs early after surgery, originating from the plasma and/or cells that invade from the blood and/or adjacent tissues, rather than from cells residing in the VG at the time of surgery. Gulkarov et al. showed that CRP protein was deposited in canine VGs shortly after surgery, but found no evidence of CRP gene expression within VGs [7]. Similarly, CRP protein deposition has been detected in the wall of human VGs [6, 27]. Jabs et al. reported RT-PCR evidence of CRP gene expression in VGs retrieved from patients undergoing a second CABG operation [6]. This study examined local CRP gene expression in end-stage, arteriosclerotic VGs, as opposed to within VGs undergoing the very early phases of remodeling, which is more relevant to early VG thrombosis. Jabs et al. also found evidence of CRP gene expression in saphenous veins harvested from humans undergoing CABG. In contrast, Wilson et al. did not detect CRP gene expression in saphenous veins of 28 patients undergoing CABG [28]. Therefore, our findings regarding the origin of CRP in VGs are generally consistent with available large animal and human data.
A main objective of our study was to examine the effect of transgenic expression of human CRP on TF and PAI-1 gene expression and protein concentration in VGs. We focused on these factors because they are critical pro-thrombotic molecules, and because in vitro cell culture studies have shown that CRP up-regulates TF expression in endothelial cells and SMCs [8-10], and PAI-1 expression in endothelial cells [11, 12]. Establishing whether these effects are also exerted in vivo is essential to an adequate understanding of CRP's vascular biological effects. Our data suggest that human CRP up-regulates TF expression in murine VGs. The effect was modest at 7 days, producing a significant increase in TF gene expression, though the higher TF immunostaining in VGs from CRP-Tg mice did not achieve statistical significance compared to controls. At 28 days, there was a strong increase in TF immunostaining in CRP-Tg VGs compared to WT controls, though the higher TF gene expression in CRP-Tg VGs did not achieve statistical significance compared to controls. These findings raise the possibility that a significant portion of the TF deposited in VGs may originate from a systemic source, possibly from invading macrophages [22]. Supporting this hypothesis, we showed that purified human CRP stimulated macrophage TF expression, consistent with previous studies [29, 30], and macrophage migration in vitro. We also showed that macrophage infiltration into VGs was greater in CRP-Tg mice compared to controls.
We found no apparent effect of human CRP on PAI-1 gene expression in VGs 7 days after surgery. However, PAI-1 concentration in the VG wall was significantly increased at this time point in CRP-Tg VGs compared to WT controls. In addition, plasma PAI-1 concentration was significantly increased in CRP-Tg mice 7 days after surgery. Our data and published studies revealed substantial endothelial cell denudation in VGs at this time point [15, 31], raising the possibility that PAI-1 may transition from the plasma into the VG wall early after surgery due to loss of the endothelial cell barrier. At 28 days after surgery, we observed a modest decrease in PAI-1 gene expression in VGs of CRP-Tg mice compared to controls. At this time point there was no significant difference in PAI-1 protein concentration in the sub-endothelial neointima of CRP-Tg vs. WT mice. Overall, our data suggest that human CRP does not increase PAI-1 gene expression in the SMC-rich layer of the neointima of VGs undergoing the early stages of remodeling, which differs from its effects on TF. However, PAI-1 immunostaining within the vascular endothelium (which had recovered by 28 days) was significantly increased in VGs of CRP-Tg mice compared to WT VGs, suggesting that the effect of CRP on endothelial PAI-1 expression observed in vitro extends to VGs in vivo.
Previous studies have identified possible mechanisms by which CRP up-regulates TF and PAI-1 expression. CRP increases TF expression in SMCs by pathways involving reactive oxygen species generation, Fcγ receptor IIIa, p44/42 MAP kinase, and nuclear factor kappa-B (NF-κB) [9, 10]. CRP-induced PAI-1 expression in endothelial cells appears to be mediated by oxidative stress and p38 intracellular signaling [12], as well as by stabilization of PAI-1 mRNA [11].
Our second major objective was to determine if human CRP enhances fibrin formation in VGs. Consistent with this hypothesis, we observed increased fibrin deposition after mechanical injury in VGs of CRP-Tg mice compared to those of WT mice, which supports a causal role of enhanced CRP expression in early VG thrombosis. We also found increased spontaneous intramural fibrin deposition 28 days after surgery in VGs of CRP-Tg mice compared to controls. Although intramural fibrin deposition is distinct from intravascular thrombosis, fibrin formation within the VG wall occurs and has been implicated in the development of VG disease [22]. While we observed enhanced expression of TF and PAI-1 in VGs in response to transgenic expression of human CRP, we cannot determine from our data which of these factors is dominant in producing the fibrin-enhancing effect. Factors other than TF and PAI-1 are also likely contributing [32-34]. Additional studies will be necessary to address these issues. A previous study demonstrated increased platelet-fibrin thrombosis after photochemical arterial injury in human CRP-Tg mice [35]. However, using the ferric chloride model, which generates occlusive, platelet-rich thrombi, we did not discern a significant difference in the time required to form an occlusive thrombus in VGs of CRP-Tg vs. WT mice. This result does not negate our vascular fibrin accumulation data, as different forms of vascular injury can activate distinct pro-thrombotic pathways, only some of which may be regulated by a particular factor. For example, the effect of glycoprotein VI on thrombosis in mice is evident in the ferric chloride model, yet undetectable in response to laser injury [36]. Furthermore, it is possible that procoagulant proteins induced in the vascular wall by human CRP could be inactivated by the intense oxidative stress induced by ferric chloride, possibly resulting in loss of detection of a real procoagulant effect. An advantage of the mechanical injury model that we employed is that it is probably more relevant to human thrombosis than the ferric chloride model.
In summary, we employed a transgenic mouse model to test the hypothesis that human CRP promotes early VG thrombosis. We showed that CRP up-regulates the concentration of TF and PAI-1 in VGs, and that these changes are accompanied by increased VG fibrin formation. These results support the hypothesis that CRP promotes early VG failure by activating prothrombotic pathways. Overall, our study suggests that CRP is not only a marker of VG thrombosis, but is also mechanistically involved in the process by enhancing TF and PAI-1 expression.
Supplementary Material
Acknowledgments
None.
Funding Sources: This work was supported by a Merit Review Award from the Department of Veterans Affairs, NIH grants HL57346 and HL95951, and a grant from the Missouri Life Sciences Research Board.
Footnotes
Disclosures: The authors state that they have no conflict of interest.
Addendum: Y. Ji designed the study, performed experiments, analyzed results, and wrote the manuscript. T. L. Strawn, A. W. Lohman, and P. M. Fish, and J. Wu performed experiments, analyzed results, prepared figures, and edited the manuscript. A. J. Szalai contributed substantially to experimental design, data interpretation, and manuscript editing. W. P. Fay designed experiments, analyzed and interpreted data, and wrote the manuscript.
References
- 1.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–31. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 2.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aouifi A, Piriou V, Blanc P, Bouvier H, Bastien O, Chiari P, Rousson R, Evans R, Lehot JJ. Effect of cardiopulmonary bypass on serum procalcitonin and C-reactive protein concentrations. Br J Anaesthesia. 1999;83:602–7. doi: 10.1093/bja/83.4.602. [DOI] [PubMed] [Google Scholar]
- 4.Hedman A, Larsson PT, Alam M, Wallen NH, Nordlander R, Samad BA. CRP, IL-6 and endothelin-1 levels in patients undergoing coronary artery bypass grafting. Do preoperative inflammatory parameters predict early graft occlusion and late cardiovascular events? Int J Cardiol. 2007;120:108–14. doi: 10.1016/j.ijcard.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Balciunas M, Bagdonaite L, Samalavicius R, Griskevicius L, Vuylsteke A. Pre-operative high sensitive C-reactive protein predicts cardiovascular events after coronary artery bypass grafting surgery: a prospective observational study. Ann Card Anaesthesia. 2009;12:127–32. doi: 10.4103/0971-9784.53442. [DOI] [PubMed] [Google Scholar]
- 6.Jabs WJ, Theissing E, Nitschke M, Bechtel JFM, Duchrow M, Mohamed S, Jahrbeck B, Sievers HH, Steinhoff J, Bartels C. Local generation of C-reactive protein in diseased coronary artery venous bypass grafts and normal vascular tissue. Circulation. 2003;108:1428–31. doi: 10.1161/01.CIR.0000092184.43176.91. [DOI] [PubMed] [Google Scholar]
- 7.Gulkarov I, Pintucci G, Bohmann K, Saunders PC, Sullivan RF, Ferrari G, Mignatti P, Galloway AC. Mechanisms of C-reactive protein up-regulation in arterialized vein grafts. Surgery. 2006;139:254–62. doi: 10.1016/j.surg.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Wang J, Yao Y, Yuan W, Kong M, Lin Y, Geng D, Nie R. CRP regulates the expression and activity of tissue factor as well as tissue factor pathway inhibitor via NF-kappaB and ERK 1/2 MAPK pathway. FEBS Lett. 2009;583:2811–8. doi: 10.1016/j.febslet.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 9.Cirillo P, Golino P, Calabro P, Cali G, Ragni M, De Rosa S, Cimmino G, Pacileo M, De Palma R, Forte L, Corigliano FG, Angri V, Spagnuolo R, Nitsch L, Chiariello M. C-reactive protein induces tissue factor expression and promotes smooth muscle and endothelial cell proliferation. Cardiovasc Res. 2005;68:47–55. doi: 10.1016/j.cardiores.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Wu J, Stevenson MJ, Brown JM, Grunz EA, Strawn TL, Fay WP. C-Reactive protein enhances tissue factor expression by vascular smooth muscle cells. Mechanisms and in vivo significance. Arterioscler Thromb Vasc Biol. 2008;28:698–704. doi: 10.1161/ATVBAHA.107.160903. [DOI] [PubMed] [Google Scholar]
- 11.Devaraj S, Xu DY, Jialal I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells. Circulation. 2003;107:398–404. doi: 10.1161/01.cir.0000052617.91920.fd. [DOI] [PubMed] [Google Scholar]
- 12.Chen C, Nan B, Lin P, Yao Q. C-reactive protein increases plasminogen activator inhibitor-1 expression in human endothelial cells. Thromb Res. 2008;122:125–33. doi: 10.1016/j.thromres.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciliberto G, Arcone R, Wagner EF, Ruther U. Inducible and tissue-specific expression of human C-reactive protein in transgenic mice. EMBO J. 1987;6:4017–22. doi: 10.1002/j.1460-2075.1987.tb02745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szalai AJ, van Ginkel FW, Dalrymple SA, Murray R, McGhee JR, Volanakis JE. Testosterone and IL-6 Requirements for Human C-Reactive Protein Gene Expression in Transgenic Mice. J Immunol. 1998;160:5294–9. [PubMed] [Google Scholar]
- 15.Zou Y, Dietrich H, Hu Y, Metzler B, Wick G, Xu Q. Mouse model of venous bypass graft arteriosclerosis. Am J Pathol. 1998;153:1301–10. doi: 10.1016/S0002-9440(10)65675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji Y, Strawn TL, Grunz EA, Stevenson MJ, Lohman AW, Lawrence DA, Fay WP. Multifaceted Role of Plasminogen Activator Inhibitor-1 in Regulating Early Remodeling of Vein Bypass Grafts. Arterioscler Thromb Vasc Biol. 2011;31:1781–7. doi: 10.1161/ATVBAHA.111.228767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-Delta Delta CT Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Reinhardt C, von B, Manukyan D, Grahl L, Lorenz M, Altmann B, Dlugai S, Hess S, Konrad I, Orschiedt L, Mackman N, Ruddock L, Massberg S, Engelmann B. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008;118:1110–22. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fay WP, Parker AC, Ansari MN, Zheng X, Ginsburg D. Vitronectin inhibits the thrombotic response to arterial injury in mice. Blood. 1999;93:1825–30. [PubMed] [Google Scholar]
- 20.Zahedi K, Tebo JM, Siripont J, Klimo GF, Mortensen RF. Binding of human C-reactive protein to mouse macrophages is mediated by distinct receptors. J Immunol. 1989;142:2384–92. [PubMed] [Google Scholar]
- 21.Bisoendial RJ, Kastelein JJP, Levels JHM, Zwaginga JJ, van den Bogaard B, Reitsma PH, Meijers JCM, Hartman D, Levi M, Stroes ESG. Activation of inflammation and coagulation after infusion of C-reactive protein in humans. Circ Res. 2005;96:714–6. doi: 10.1161/01.RES.0000163015.67711.AB. [DOI] [PubMed] [Google Scholar]
- 22.Stark VK, Warner TF, Hoch JR. An ultrastructural study of progressive intimal hyperplasia in rat vein grafts. J Vasc Surg. 1997;26:94–103. doi: 10.1016/s0741-5214(97)70152-6. [DOI] [PubMed] [Google Scholar]
- 23.Hoch JR, Stark VK, van Rooijen N, Kim JL, Nutt MP, Warner TF. Macrophage depletion alters vein graft intimal hyperplasia. Surgery. 1999;126:428–37. [PubMed] [Google Scholar]
- 24.Stein MP, Mold C, Du Clos TW. C-reactive protein binding to murine leukocytes requires Fc gamma receptors. J Immunol. 2000;164:1514–20. doi: 10.4049/jimmunol.164.3.1514. [DOI] [PubMed] [Google Scholar]
- 25.Xing D, Hage FG, Chen YF, McCrory MA, Feng W, Skibinski GA, Majid-Hassan E, Oparil S, Szalai AJ. Exaggerated neointima formation in human C-reactive protein transgenic mice is IgG Fc receptor type I (FcgammaRI)-dependent. Am J Pathol. 2008;172:22–30. doi: 10.2353/ajpath.2008.070154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hage FG, Oparil S, Xing D, Chen YF, McCrory MA, Szalai AJ. C-reactive protein-mediated vasculariInjury requires complement. Arterioscler Thromb Vasc Biol. 2010;30:1189–95. doi: 10.1161/ATVBAHA.110.205377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho KJ, Owens CD, Longo T, Sui XX, Ifantides C, Conte MS. C-reactive protein and vein graft disease: evidence for a direct effect on smooth muscle cell phenotype via modulation of PDGF receptor-beta. Am J Physiol Heart Circ Physiol. 2008;295:H1132–H1140. doi: 10.1152/ajpheart.00079.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson AM, Swan JD, Ding H, Zhang Y, Whitbourn RJ, Gurry J, Yii M, Wilson AC, Hill M, Triggle C, Best JD, Jenkins AJ. Widespread vascular production of C-reactive protein (CRP) and a relationship between serum CRP, plaque CRP and intimal hypertrophy. Atherosclerosis. 2007;191:175–81. doi: 10.1016/j.atherosclerosis.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 29.Nakagomi A, Freedman SB, Geczy CL. Interferon-γ and lipopolysaccharide potentiate monocyte tissue factor induction by C-reactive protein: Relationship with age, sex, and hormone replacement treatment. Circulation. 2000;101:1785–91. doi: 10.1161/01.cir.101.15.1785. [DOI] [PubMed] [Google Scholar]
- 30.Devaraj S, Dasu MR, Singh U, Rao LV, Jialal I. C-reactive protein stimulates superoxide anion release and tissue factor activity in vivo. Atherosclerosis. 2009;203:67–74. doi: 10.1016/j.atherosclerosis.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kockx M, Cambier B, Bortier H, Meyer G, Cauwelaert P. The modulation of smooth muscle cell phenotype is an early event in human aorto-coronary saphenous vein grafts. Vichows Archiv A Pathol Anat. 1992;420:155–62. doi: 10.1007/BF02358807. [DOI] [PubMed] [Google Scholar]
- 32.Danenberg HD, Kantak N, Grad E, Swaminathan RV, Lotan C, Edelman ER. C-Reactive protein promotes monocyte-platelet aggregation: An additional link to the inflammatory-thrombotic intricacy. Eur J Haem. 2007;78:246–52. doi: 10.1111/j.1600-0609.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 33.Yaron G, Brill A, Dashevsky O, Yosef-Levi IM, Grad E, Danenberg HD, Varon D. C-reactive protein promotes platelet adhesion to endothelial cells: a potential pathway in atherothrombosis. British Journal of Haematology. 2006;134:426–31. doi: 10.1111/j.1365-2141.2006.06198.x. [DOI] [PubMed] [Google Scholar]
- 34.Devaraj S, Kumaresan PR, Jialal I. C-Reactive protein induces release of both endothelial microparticles and circulating endothelial cells In vitro and in vivo: Further evidence of endothelial dysfunction. Clin Chem. 2011;57:1757–61. doi: 10.1373/clinchem.2011.169839. [DOI] [PubMed] [Google Scholar]
- 35.Danenberg HD, Szalai AJ, Swaminathan RV, Peng L, Chen Z, Seifert P, Fay WP, Simon DI, Edelman ER. Increased thrombosis after arterial injury in human C-reactive protein-transgenic mice. Circulation. 2003;108:512–5. doi: 10.1161/01.CIR.0000085568.13915.1E. [DOI] [PubMed] [Google Scholar]
- 36.Dubois C, Panicot-Dubois L, Merrill-Skoloff G, Furie B, Furie BC. Glycoprotein VI–dependent and –independent pathways of thrombus formation in vivo. Blood. 2006;107:3902–6. doi: 10.1182/blood-2005-09-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


