Abstract
Objective
Progressive multifocal leukoencephalopathy (PML), caused by JC virus (JCV), can occur in patients receiving natalizumab for multiple sclerosis (MS). JCV detection by quantitative polymerase chain reaction (qPCR) in cerebrospinal fluid (CSF), or brain biopsy, is required for probable or definite diagnosis of PML. However, in some patients only low levels of JCV DNA (<100 copies/ml) are present in CSF, making the diagnosis challenging. Our objective was to assess the complementary value of a CSF JCV antibody index (AIJCV) in the diagnosis of natalizumab-associated PML.
Methods
AIJCV was assessed in 37 cases of natalizumab-associated PML and 89 MS-patients treated with natalizumab without PML. Sera and CSF were tested in a capture enzyme-linked immunosorbent assay, using JCV-VP1 fused to glutathione S-transferase as antigen. Albumin levels and total immunoglobulin G concentration were determined by immunonephelometry, and the AIJCV was calculated as published.
Results
Twenty-six of 37 (70%) patients with natalizumab-associated PML exhibited an AIJCV > 1.5, whereas this was seen in none of the controls (p < 0.0001). At time of the first positive qPCR for JCV DNA, 11 of 20 (55%) patients with natalizumab-associated PML had an AIJCV > 1.5. JCV DNA levels of <100 copies/ml were seen in 14 (70%) of these 20 patients, of whom 8 (57%) demonstrated an AIJCV > 1.5.
Interpretation
Determination of the AIJCV could be an added tool in the diagnostic workup for PML and should be included in the case definition of natalizumab-associated PML.
Natalizumab (NAT) is an approved therapy for relapsing multiple sclerosis (MS). However, a substantial complication in patients treated with NAT for MS is progressive multifocal leukoencephalopathy (NAT-PML), a demyelinating lytic central nervous system (CNS) infection by JC virus (JCV).1–3 Variable clinical presentation and imaging features, similarity of clinical signs of PML to MS relapse activity, and the lack of noninvasive diagnostic tools that confirm PML in patients with lesions suspicious for PML on brain magnetic resonance imaging (MRI) complicate the early recognition of cases of NAT-PML. The diagnostic steps in PML workup include clinical examination, MRI, and quantitative polymerase chain reaction (qPCR) for detection of JCV DNA in cerebrospinal fluid (CSF). However, sensitivity of JCV DNA PCR in CSF for diagnosis of PML is variable, ranging from 60 to 95%.4,6,7 Most commercial laboratories are only able to detect JCV DNA in quantities >200 copies/ml CSF, whereas the laboratory at the NIH has a limit of 10 copies JCV DNA/ml.6 However, the significance of very low copy numbers (< 100 copies/ml) is not entirely clear, as these have previously been noted in 2 of 515 CSF samples from patients without apparent clinical or radiographic signs of PML.8 Despite the use of ultrasensitive protocols and continuous efforts to improve the methodology,9 patients with NAT-PML can have repetitively undetectable JCV DNA in CSF,10–12 and frequently have a JCV DNA level of <100 copies/ml CSF at time of diagnosis.13–14 This can lead to delayed diagnosis in some patients,15 or to a brain biopsy to confirm the diagnosis in others. As of July 2011, in 9.2% of German PML cases associated with monoclonal antibody therapy, brain biopsy has been performed to confirm the clinical suspicion of PML.4
Thus, there is a need for additional diagnostic tests that allow the diagnosis of probable or definite PML in the setting of clinical or imaging suspicion of possible PML. The detection of a marked rise in anti-JCV immunoglobulin G (IgG) antibodies in the CSF with evidence for intrathecal production has been observed in cases of NAT-PML, 10,11 and has previously been reported in non-MS PML cases.16,17 The aim of our study was to assess whether the CSF JCV antibody index (AIJCV) as a measure of intrathecal synthesis of anti-JCV antibodies could add to the diagnosis of NAT-PML.
Subjects and Methods
Patients
Paired CSF and serum samples from patients with NAT-PML at or after diagnosis of PML that had been sent to the Institute for Virology at Heinrich Heine University, Düsseldorf, Germany, and the Laboratory of Molecular Medicine and Neuroscience, National Institute of Neurological Disorders and Stroke, NIH, Bethesda, Maryland for the purpose of JCV DNA analysis were included in this study. A description of the clinical and radiographic findings of the patients was obtained from the treating physician.
CSF–serum pairs of non-PML patients with relapsing–remitting MS treated with NAT (NAT controls) available from German and Swedish19,20 pharmacovigilance studies served as controls.
The local ethics committee (Ethics Commission of Heinrich Heine University, Düsseldorf, protocol number 3315) approved the study and waved the requirement for written informed consent for the retrospective analysis of the stored samples at the Institute for Virology, Düsseldorf.
Anti-JCV Antibody Detection, Calculation of CSF JCV Antibody Index, and JCV DNA Detection
Sera and CSF were tested in a recently published species-specific capture enzyme-linked immunosorbent assay using JCV-VP1 fused to glutathione S-transferase as antigen.21,22 For the determination of JCV-VP1–specific IgG antibodies, sera were tested at 1:60 and 1:180 dilutions and CSF at 1:3 and 1:9 dilutions. Highly reactive sera and CSF with an OD450 ≥ 1.5 at the 1:180 and 1:9 dilutions, respectively, were further diluted. The antibody reactivity was assessed in arbitrary units (AU) by the use of a standard curve obtained from a serial dilution of human polyclonal immunoglobulin at stable concentrations as published.22 AUs were multiplied by the dilution factors for dilutions within the linear part of the standard curve (OD450 < 1.5) to obtain absolute AU values. Total IgG and albumin concentrations in blood and CSF were determined on a BN ProSpec analyzer using commercially available immunonephelometric assays (Siemens Health Care Diagnostics, Eschborn, Germany). Nephelometric measurements were performed in the central laboratory of the University Hospital Düsseldorf according to the manufacturer’s instructions.
The AIJCV was calculated as published.23 Briefly, Qlim represents the blood-derived IgG fraction in CSF, which is calculated from the individual albumin quotient (QAIb = AIbCSF/AIbserum) as follows:. . If . If . Consistent with the literature, an AIJCV of >1.5 was considered as evidence for intrathecal antibody synthesis.16,23
qPCR for the detection of JCV DNA was performed as published,24 amplifying a 78bp product in the large-antigen region.25,26
Statistical Analysis
Prism v6.0 (Graph Pad Software, San Diego, CA) was used for statistical analysis. To compare categorical values, chi-square test was applied; 95% confidence intervals (CIs) for proportions were calculated by modified Wald method (http://www.graphpad.com/quickcalcs/). To compare unpaired nonparametric measures, Mann–Whitney test was applied. To assess the correlation between nonparametric values (JCV DNA levels and AIJCV), Spearman r correlation coefficients were calculated. Probability values of <0.05 were considered significant.
Results
Patient Characteristics
A total of 37 patients with NAT-PML were included in this study, comprising 30 cases from Germany, 1 from the Netherlands, 1 from Sweden, and 5 from the United States. In 54% (20 of 37) of these cases, a CSF–serum pair at time of diagnosis of PML was available, as generally defined by the date of the first PCR positive for JCV DNA. In the remaining 46% (17 of 37) of the cases, only samples obtained after the initial PML diagnosis were tested.
A total of 89 NAT-treated patients without PML from Germany (n = 48) and Sweden (n = 41) served as controls. Compared to these NAT controls, our NAT-PML patients were older (mean age = 45 vs 40 years, p = 0.012) and had a longer median duration of therapy with NAT (44 vs 14 months, p < 0.0001; Table 1).
TABLE 1.
Patient Characteristics
| Patient Groups |
No. of Patients |
Mean Age, yr (range) |
Median No. of Infusions (range) |
Gender, Female/Male [%] |
|---|---|---|---|---|
| NAT controls | 89 | 40 (22–61) | 14 (6–50) | 53/85 [62%] |
| German | 48 | 40 (24–61) | 24 (19–50) | 30/44 [68%] |
| Swedish | 41 | 41 (22–61) | 12 (6–14) | 23/41 [56%] |
| NAT-PML | 37 | 45 (28–63) | 44 (19–75) | 25/37 [68%] |
| After Dx | 17 | 46 (30–63) | 43 (23–66) | 12/17 [71%] |
| At Dx | 20 | 44 (28–51) | 45 (19–75) | 13/20 [65%] |
|
NAT-PML Cases at Dx |
Group |
JCV DNA in CSF, Copies/ml |
AIJVC | Comment |
| Case 12 | qPCR > 100 copies/ ml, AIJCV > 1.5 |
120 | 5.63 | Negative PCR but AIJCV = 3.60 at 3 months prior to Dx, Fig 2A |
| Case 8 | 444 | 48.78 | ||
| Case 20 | 69,716 | 7.29 | ||
| Case 19 | qPCR > 100 copies/ ml, AIJCV < 1.5 |
306 | 0.58 | |
| Case 4 | 6,930 | 0.52 | ||
| Case 9 | 11,900 | 0.71 | ||
| Case 17 | qPCR < 100 copies ml, AIJCV > 1.5 |
Indeterminate | 35.90 | Indeterminate + PCR result 2 months prior to Dx, Fig 2B |
| Case 16 | 14 | 13.66 | Negative in local reference laboratory, 14 copies/ml at NIH, Fig 2C |
|
| Case 18 | 91 | 11.33 | ||
| Case 13 | 56 | 4.46 | ||
| Case 11 | 25 | 3.72 | Indeterminate+ PCR result in local reference laboratory, 25 copies/ ml at NIH, Fig 2D |
|
| Case 3 | 1 | 3.04 | ||
| Case 6 | 20 | 1.76 | ||
| Case 5 | 31 | 2.29 | ||
| Case 1 | qPCR < 100 copies/ ml, AIJCV < 1.5 |
75 | 1.37 | |
| Case 2 | 9 | 1.30 | ||
| Case 7 | 37 | 0.09 | ||
| Case 10 | 52 | 1.03 | ||
| Case 14 | 30 | 0.39 | MRI suspicion, negative PCR at local laboratory, 30 copies/ml at NIH |
|
| Case 15 | 24 | 0.37 | ||
AIJCV = CSFJCV antibody index; CSF = cerebrospinal fluid; Dx = diagnosis; JCV = JC virus; MRI = magnetic resonance imaging; NAT = natalizumab; PML = progressive multifocal leukoencephalopathy; qPCR = quantitative polymerase chain reaction.
Anti-JCV Antibody Levels in Blood and CSF, and CSF JCV Antibody Index at Diagnosis and during NAT-Associated PML
Anti-JCV antibodies in serum were detectable in 63 of 89 (71%, 95% CI = 61–79%) NAT controls, and in all (100%) of the NAT-PML patients. The anti-JCV antibody levels were higher in serum of NAT-PML patients obtained at diagnosis of PML (median absolute AU = 12,930; range = 1,290–129,420) or later during the course of PML (median absolute AU = 91,140; range = 1,260–570,600) compared with samples of the 63 antibody-positive NAT controls (median absolute AU = 4,624, range = 1,100–38,437, p < 0.0001 for both comparisons; Fig 1A).
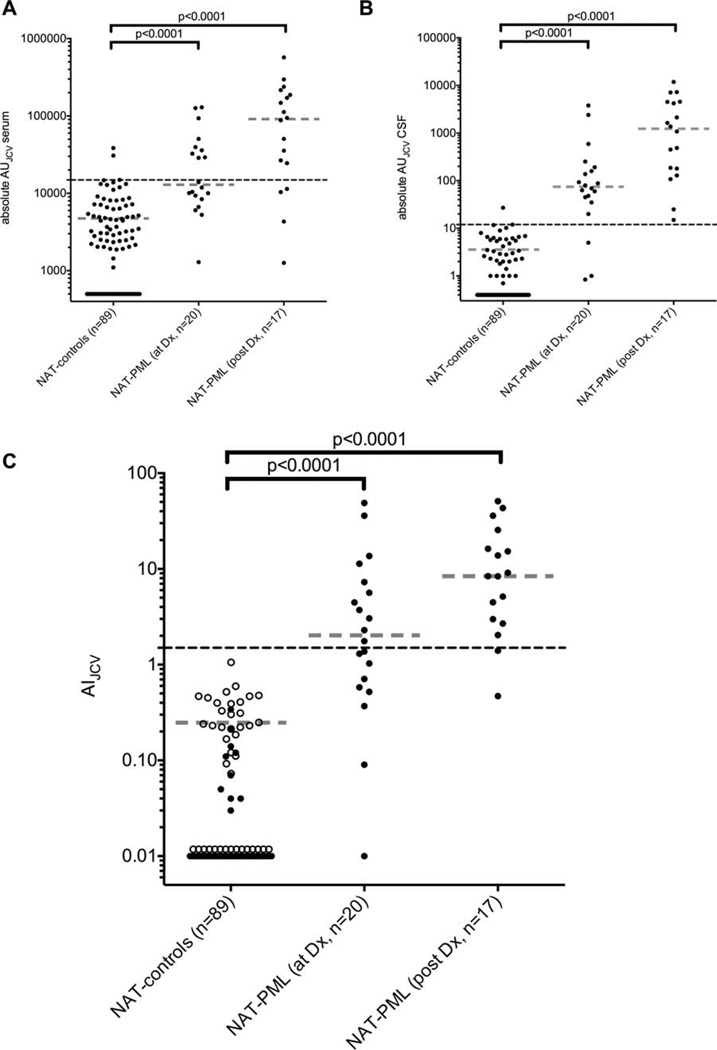
FIGURE 1.
The anti-JC virus (JCV) antibody levels in absolute arbitrary units (AU) are shown in (A) blood and (B) cerebrospinal fluid (CSF). (C) The CSFJCV antibody index (AIJCV) values of 89 non-progressive multifocal leukoencephalopathy (PML) patients with relapsing-remitting multiple sclerosis treated with natalizumab (NAT controls) from Germany (n = 48, soild circles) and Sweden (n = 41, open circles) are compared with the AIJCV values of 37 cases of NAT-associated PML (NAT-PML) at diagnosis (Dx) of PML (n = 20, prior to plasma exchange) or in samples obtained thereafter (n = 17). For all NAT-PML cases, the first available CSF-serum pair relative to the time of Dx of PML is shown. Black dashed lines indicate 95% CI of antibody-positive NAT controls (A, B) or the AIJCV cutoff at 1.5 (C); continuous lines indicate NAT controls that tested negative; gray dashed lines indicate median of patients with detectable anti-JCV antibodies.
Anti-JCV antibodies in CSF were detectable in 39 of 89 (44%, 95% CI = 34–54%) NAT controls. The anti-JCV antibody levels in CSF were higher in CSF of NAT-PML patients obtained at diagnosis of PML (median absolute AU = 74.5, range = 0.84–3,807) or later during the course of PML (median absolute AU = 1,232, range = 15–11,880) compared with samples of the 39 antibody-positive NAT controls (median absolute AU = 3.4, range = 0.7–27, p < 0.0001 for both comparisons; see Fig 1B).
AIJCV values were higher in NAT-PML patients (median AIJCV = 4.46, range = 0.09–51.04) compared to the NAT controls (median AIJCV = 0.23, range = 0.03–1.22, p < 0.0001). This was also true when analyzing samples of NAT-PML patients obtained at diagnosis of PML (median AIJCV = 2.03, range = 0.09–48.78) or later in the PML course (median AIJCV = 8.40, range = 0.47–51.04) separately (see Fig 1C).
Accordingly, 26 of 37 (70%, 95% CI = 54–83%) patients with NAT-PML showed an AIJCV > 1.5 suggestive for intrathecal anti-JCV IgG antibody synthesis, whereas this was seen in none of the 89 NAT controls (p < 0.0001). In patients with sampling available at diagnosis, 11 of 20 (55%, 95% CI = 34–74%) NAT-PML cases showed an AIJCV > 1.5 (see Table).
JCV DNA Levels at Diagnosis of PML
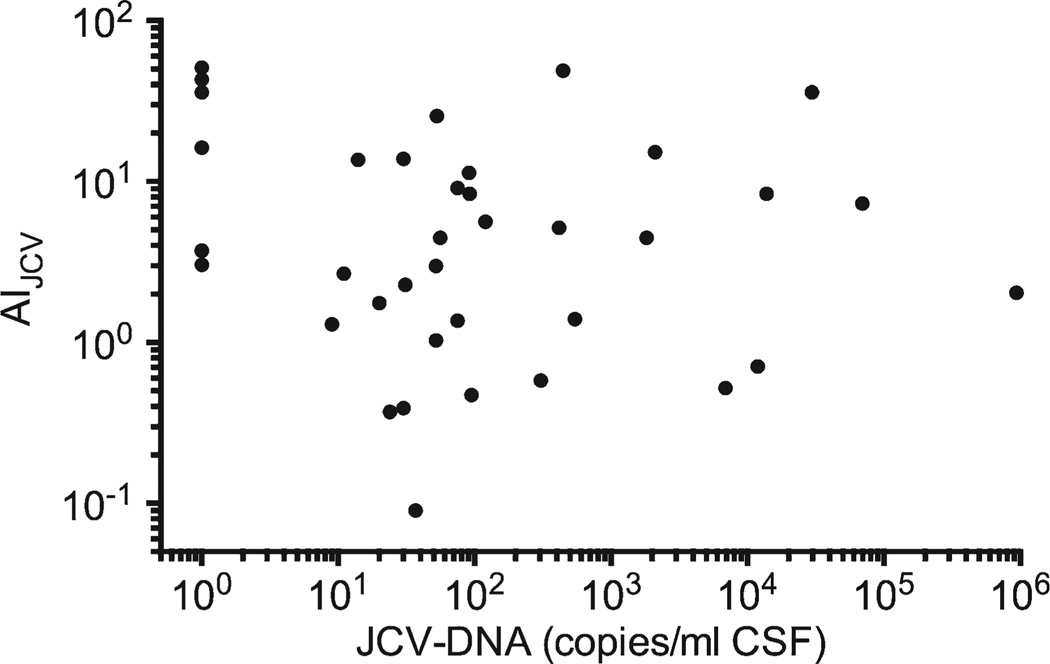
As per definition, NAT-PML patients with sampling available at diagnosis had detectable JCV DNA in their CSF by qPCR, with 1 exception repetitively showing indeterminate PCR results (Fig 2B). However, 14 (70%, 95% CI = 48–86%) of these 20 patients had JCV DNA levels of <100 copies/ml. Eight of these 14 (57%, 95% CI = 33–79%) demonstrated an AIJCV > 1.5 at time of diagnosis (see Table). No correlation was observed between the AIJCV and the molecular detection of JCV DNA by qPCR, neither when analyzing all PML cases together (Spearman r = 0.095, 95% CI = −0.415 to 0.246, Fig 3), nor when analyzing the cases with sampling at time of diagnosis separately (Spearman r = −0.056, 95% CI = −0.497 to 0.408).
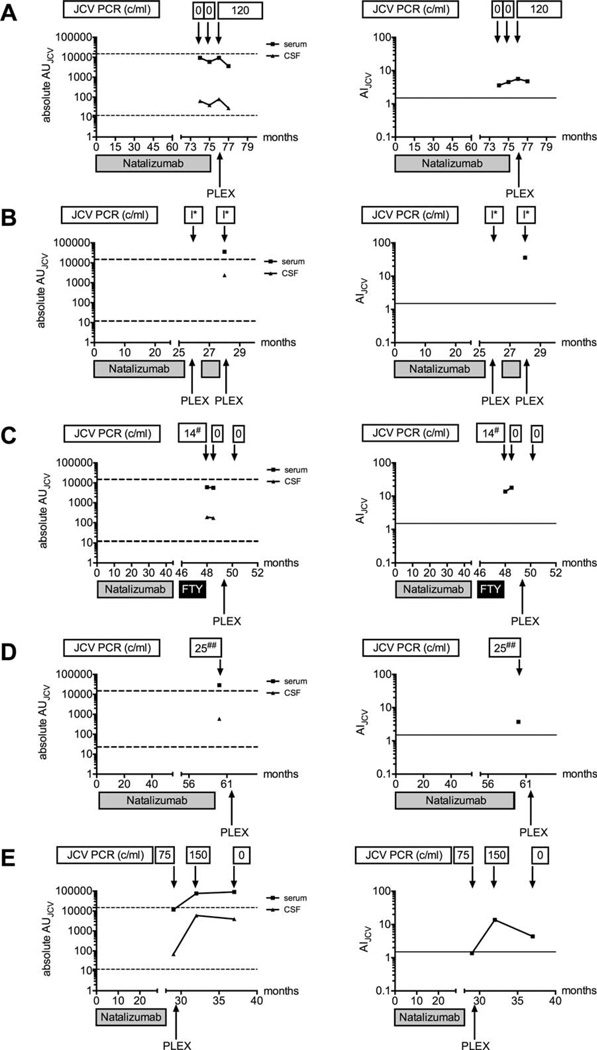
FIGURE 2.
Five cases of natalizumab-associated progressive multifocal leukoencephalopathy (PML) with high CSFJC virus (JCV) antibody index (AIJCV) values prior to or at diagnosis of PML are shown in an x–y diagram, plotting the absolute arbitrary unit (AU) values in blood and cerebrospinal fluid (CSF; left) and the AIJCV values (right) over time. PLEX marks the start with plasma exchange or immune adsorption. I* indicates indeterminate polymerase chain reaction (PCR) result at a local virology laboratory, negative at the Institute for Virology, Düsseldorf. 14# indicates 14 copies/ml CSF at the NIH, negative at the Swedish reference laboratory for JCV testing. 25## indicates 25 copies/ml CSF at the NIH, indeterminate result (1 probe positive in 2 duplicates and repeated testing) at the Institute for Virology, Düsseldorf. Dashed lines indicate 95% confidence interval of antibody-positive natalizumab controls in blood (upper line) or CSF (lower line); continuous lines indicate AIJCV cutoff at 1.5. FTY = fingolimod.
FIGURE 3.
The CSFJC virus (JCV) antibody index (AIJCV) values and the JCV DNA results by polymerase chain reaction of all 37 natalizumab-progressive multifocal leukoencephalopathy cases are shown in an x–y diagram. AIJCV values and JCV DNA level did not correlate (Spearman r = 0.095, 95% confidence interval = −0.415 to 0.246). CSF = cerebrospinal fluid.
Individual Cases That Demonstrate Potential Role of Using CSF JCV Antibody Index in Diagnosing PML in Patients Treated with NAT
In 1 of our cases of NAT-PML, clinical and MRI findings raised the suspicion of PML, prompting CSF analysis for JCV DNA, which did not detect viral DNA at 2 time points. However, in this patient definite diagnosis of PML was established 3 months later, when 120 copies/ml of JCV DNA were detected on repeat CSF testing at the Institute for Virology in Düsseldorf. Compatible with clinical suspicion of an earlier start of PML, the retrospective analysis of stored CSF-serum pairs already displayed an elevated and increasing AIJCV 3 and 2 months prior to the first positive detection of JCV DNA in CSF by qPCR (see Fig 2A).
In another patient on NAT, a delayed diagnosis of PML was based on clinical and MRI changes, 2 indeterminate positive JCV DNA PCR results from a local virology laboratory (despite repetitive negative JCV DNA PCR results at the Institute for Virology, Düsseldorf), and evidence for pronounced intrathecal anti-JCV antibody synthesis from our study. As the clinical deterioration was initially judged to be new MS disease activity— a diagnosis influenced by the inconclusive PCR results and MS-like imaging appearance of the lesion—plasma exchange (PLEX) as escalation treatment for relapse and 1 additional infusion of NAT therapy were given in this case prior to diagnosis of PML (see Fig 2B).
A third case was diagnosed with NAT-PML 4 months after cessation of NAT and switching to fingolimod therapy. Whereas JCV DNA in CSF was repetitively found negative at the Swedish reference laboratory for JCV diagnostics, 14 copies/ml of JCV DNA in CSF were detected at the NIH at the time of the first lumbar puncture. At the same time, clear evidence of intrathecal anti-JCV antibody synthesis was noted, which increased over time, supporting diagnosis of NAT-PML (see Fig 2C).
In another patient, clinical and MRI suspicion led to JCV DNA testing in CSF, resulting in probable PML based on an indeterminate positive PCR result (repetitively positive probe in 1 of 2 duplicates; tested at the Institute for Virology, Düsseldorf). The definite diagnosis of PML was based on the detection of 25 copies/ml of JCV DNA in CSF at the NIH, and clear evidence for intrathecal anti-JCV IgG production from our study (see Fig 2D).
In a fifth case, diagnosis of definite PML was based on the detection of 75 copies of JCV-DNA in CSF. The AI-JCV of 1.37 was just below the cut-off at 1.5, but increased in follow-up samples, supporting the diagnosis of natalizumab-associated PML (see Fig 2E).
Discussion
This is a unique study that addresses the potential value of the intrathecal anti-JCV IgG antibody synthesis for diagnosis of NAT-PML. Here we demonstrate that a vast majority of NAT-PML patients (92%, 95% CI = 78– 98%) reveal anti-JCV antibody levels in CSF with values outside the 95% CI of antibody-positive NAT controls (see Fig 1). These elevated anti-JCV antibody levels in CSF could be explained by intrathecal antibody synthesis, a disturbance of the blood–CSF barrier at or after onset of PML, or by diffusion of antibodies across the blood–CSF barrier in consequence of high serological responses.
To exclude pure diffusion and disturbance of blood–CSF barrier function as a putative explanation of increased levels of anti-JCV antibodies in CSF in cases of PML, the JCV-specific CSF antibody index (AIJCV) was calculated. In the first samples available from our NAT-PML patients, 26 of 37 (70%, 95% CI = 54–83%) had an AIJCV > 1.5 as clear evidence for intrathecal synthesis. A key question with regard to the potential diagnostic value of the AIJCV is how early this intrathecal immune response against JCV can be detected, and how specific it is. Within our study, we observed a specificity of 100% (95% CI = 95–100%) in diagnosis of PML in MS patients treated with NAT, as none of the 89 NAT controls from 2 different cohorts (Germany, Sweden) displayed an AIJCV > 1.5. Sensitivity at time of first positive qPCR was around 55% (95% CI = 34–74%), with 11 of 20 NAT-PML patients showing a pathological AIJCV. In 19 of these 20 patients, samples were obtained prior to PLEX or immune adsorption (IA), whereas only 1 patient (shown in Fig 2B) had received PLEX for a suspected MS relapse 8 weeks before and a second cycle the day before diagnosis of PML. This is crucial, because PLEX may impact antibody levels, including JCV-specific antibodies, with unknown effects on the AIJCV. Immediately following PLEX or IA, anti-JCV antibodies in blood have been shown to be decreased,27 and in the subsequent weeks anti-JCV antibodies in blood may increase14. Hence, until its effects on the AIJCV are better understood, it would be desirable to obtain blood and CSF samples prior to PLEX.
Diagnosis of NAT-PML can be complicated by clinical and/or imaging findings that are mistaken for MS relapse activity. The lack of detection of JCV DNA can be attributed to lower levels of sensitivity of a qPCR assay and sample integrity. Around 70% of the NAT-PML cases reported in this article showed JCV DNA copy numbers of <100/ml CSF at the time of diagnosis. The retrospective design of our study and a potential sampling bias may have contributed to these numbers. Nevertheless, for these cases additional confirmatory diagnostic tools appear to be required to ascertain the diagnosis of PML, and not to miss NAT-PML cases, or delay appropriate management of possible PML. Notably, no correlation was noted between DNA copy numbers in CSF and the AIJCV. Hence, at around 57% (95% CI = 33–79%) the sensitivity of the AIJCV at diagnosis of PML remained unchanged in the subgroup of patients with JCV DNA copy numbers of <100/ml, arguing for an additional diagnostic value of this test.
Considering the invasive nature of CSF diagnostics required for determining the AIJCV, this technique, like the detection of JCV DNA in CSF, will be unsuitable for PML risk prediction. Future strategies in this respect might include MRI screening,28–30 assessment of JCV-specific T-cell responses,31–33 JCV DNA in serum or peripheral blood cells,25,26,34,35 or cellular surface phenotyping.36 However, none of these tools has yet been prospectively validated. The only currently established risk factors for PML development during therapy with NAT are treatment duration with NAT for >2 years, prior immunosuppressive therapy, and the presence of anti-JCV antibodies in blood.3 Our data obtained in the present study on anti-JCV antibody levels in blood are in line with previous publications on high and increasing levels prior to, at, and after diagnosis of PML (see Figs 1A, 2).19,37 Nine of 20 (45%, 95% CI = 26–66%) of our patients at diagnosis of PML had absolute AU values in blood outside the 95% CI of our anti-JCV antibody-positive control group. This might support efforts to further stratify patients at risk of PML by considering the level of the anti-JCV antibody response. “Index values”—not to be confused with the CSF antibody index (AIJCV) presented in this study—from a commercially available protocol to quantify anti-JCV antibodies in blood are currently being evaluated for this purpose38. However, until such strategies have been further validated, vigorous clinical monitoring, determination of the anti-JCV antibody status, and regular MRI scanning are mandatory during NAT therapy for MS to minimize the risk.
As a clinical consequence of the results of our study, we propose the determination of CSF AIJCV in addition to JCV DNA PCR in CSF in cases of possible or probable PML (based on clinical, MRI, and/or JCV DNA PCR findings) in patients at risk.6 For the AIJCV, no additional invasive procedure is required, and the AIJCV can be determined from patient material (CSF, peripheral blood) routinely acquired at the time of lumbar puncture for the purpose of JCV DNA detection. Prerequisite only is the availability of a serological method to reliably quantify anti-JCV IgG antibodies in serum and CSF.22 By assessing the intrathecally synthesized JCV-specific IgG fraction, the compartmentalized humoral immune response to the causative agent of PML is studied. Interestingly, cases of NAT-PML at diagnosis vary with regard to MRI lesion extension (unilobar, multilobar, widespread) and signs of inflammation on MRI (contrast enhancement).39 Also, viral JCV DNA turnover and the level of the adaptive immune response in the CNS appear to be variable, as noted previously in patients with HIV-associated PML,40 and in our study for NAT-PML cases; although a proportion of our cases already show a strong intrathecal immune response at diagnosis but low viral copy numbers, a fraction of patients show high copy numbers while lacking a measurable intrathecal antibody response. It is tempting to speculate that in some cases of PML during therapy with a selective immunosuppressant such as NAT—possibly influenced by viral and host factors and/or the PML lesion location relative to the CSF drainage—strong adaptive immunity partly controls JCV replication, resulting in high AIJCV values despite the lack of JCV DNA in CSF, whereas in other cases the lack of a strong adaptive immunity results in high viral copy numbers. As such, an approach in the diagnostic workup that covers both the molecular detection of JCV DNA by qPCR and a measure of the adaptive immune response against JCV in the CNS appears rational. Based on our present data, it may seem plausible to determine the AIJCV simultaneously with the detection of JCV DNA by PCR in CSF in all cases of possible NAT-PML based on clinical and MRI suspicion. Patients with no JCV DNA in the CSF, but an AIJCV of >1.5, may be classified as probable NAT-PML. This may allow earlier therapy for PML in a proportion of patients, and avoidance of brain biopsy in some of the initially PCR-negative cases of NAT-PML. Clearly, further independent prospective confirmation is warranted to better define the diagnostic value of the AIJCV in patients treated with NAT.
Acknowledgment
This work was supported by a local faculty grant (Research Commission of Heinrich Heine University, Düsseldorf [HHU]; O.A.) and a grant from the German Ministry for Education and Research (BMBF; German Competence Network MS [KKNMS], Natalizumab Pharmacovigilance Study, 01GI1002; B.C.K.). C.W. was supported by a European Committee for Treatment and Research in MS fellowship stipend. The research leading to these results received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement No. 115303, resources of which are composed of a financial contribution from the European Union’s Seventh Framework Program (FP7/2007–2013) and European Federation of Pharmaceutical Industries and Associations companies’ in-kind contribution (to H.P.H., B.C.K, C.W.). The MS Center at the Department of Neurology, HHU is supported by the Walter and Use Rose Foundation. R.Hoh. was supported by the German Research Foundation (DFG CRC TRR128, Z2) and BMBF (KKNMS). M.S. is supported by the Niedersachsen Research Network on Neuroinfectiology of the Ministry of Science and Culture of Lower Saxony.
Footnotes
Potential Conflicts of Interest
T.D.: travel expenses, Novartis Pharma, Genzyme. R.Hoe.: grant support, Biogen Idec, Novartis. R.G.: speaker’s honoraria, grant support, board honoraria, Biogen Idec, Bayer, Teva, Novartis, Merck Serono. T.K.: travel expenses, personal compensation, Bayer Healthcare, Teva Pharma, Merck Serono, Novartis, Sanofi-Aventis, Biogen Idec; grant support, Novartis Pharma. M.S.: grant support, Biogen Idec, Novartis, Bayer Healthcare, Teva; personal fees, Biogen Idec, Novartis, Bayer Healthcare, Teva, Sanofi-Aventis, Baxter, CSL Behring, Grifols. R.Hoh.: board membership, consultancy, speaking fees, Novartis, Teva, Biogen Idec, Merck Serono, Bayer Schering, Sanofi-Aventis, Genzyme; grant support, Novartis, Teva, Biogen Idec, Merck Serono, Bayer Schering, Sanofi-Aventis. T.W.: scientific advisory board, PML Consortium; personal fees, Biogen Idec, Novartis, Sanofi-Aventis. H.-P.H.: board membership, consultancy, speaking fees, Novartis, Teva, Biogen Idec, Merck Serono, Bayer Schering, Sanofi-Aventis, Genzyme; grant support, Novartis, Teva, Biogen Idec, Merck Serono, Bayer Schering, Sanofi-Aventis. M.P.W.: personal fees, Biogen Idec, Novartis. A.S.: grant support, Biogen Idec; travel support, Biogen Idec, Genzyme/Sanofi, Merck Serono; lecture honoraria, Biogen Idec, Genzyme/Sanofi, Teva, Merck Serono; advisory board, Biogen Idec, Genzyme/Sanofi, Teva, Merck Serono. T.O: grant support, personal fees, Biogen Idec, Novartis, Genzyme, Merck. B.C.K.: board membership, consultancy, speaking fees, Novartis, Teva, Biogen Idec, Merck Serono, Bayer Schering, Sanofi-Aventis, Genzyme; grant support, Novartis, Teva, Biogen Idec, Merck Serono, Bayer Schering, Sanofi-Aventis. O.A.: personal fees, Biogen Idec.
References
- 1.Clifford DB, De Luca A, Simpson DM, et al. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9:438–446. doi: 10.1016/S1474-4422(10)70028-4. [DOI] [PubMed] [Google Scholar]
- 2.Warnke C, Menge T, Hartung HP, et al. Natalizumab and progressive multifocal leukoencephalopathy: what are the causal factors and can it be avoided? Arch Neurol. 2010;67:923–930. doi: 10.1001/archneurol.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 4.Mentzer D, Prestel J, Adams O, et al. Case definition for progressive multifocal leukoencephalopathy following treatment with monoclonal antibodies. J Neurol Neurosurg Psychiatry. 2012;83:927–933. doi: 10.1136/jnnp-2012-302478. [DOI] [PubMed] [Google Scholar]
- 5.Kappos L, Bates D, Edan G, et al. Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring. Lancet Neurol. 2011;10:745–758. doi: 10.1016/S1474-4422(11)70149-1. [DOI] [PubMed] [Google Scholar]
- 6.Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80:1430–1438. doi: 10.1212/WNL.0b013e31828c2fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhaskaran A, Racsa L, Gander R, et al. Interpretation of positive molecular tests of common viruses in the cerebrospinal fluid. Diagn Microbiol Infect Dis. 2013;77:236–240. doi: 10.1016/j.diagmicrobio.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 8.lacobaeus E, Ryschkewitsch C, Gravell M, et al. Analysis of cerebrospinal fluid and cerebrospinal fluid cells from patients with multiple sclerosis for detection of JC virus DNA. Mult Scler. 2009;15:28–35. doi: 10.1177/1352458508096870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryschkewitsch CF, Jensen PN, Major EO. Multiplex qPCR assay for ultra sensitive detection of JCV DNA with simultaneous identification of genotypes that discriminates non-virulent from virulent variants. J Clin Virol. 2013;57:243–248. doi: 10.1016/j.jcv.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhle J, Gosert R, Buhler R, et al. Management and outcome of CSF-JC virus PCR-negative PML in a natalizumab-treated patient with MS. Neurology. 2011;77:2010–2016. doi: 10.1212/WNL.0b013e31823b9b27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aly L, Yousef S, Schippling S, et al. Central role of JC virus-specific CD4+ lymphocytes in progressive multi-focal leucoencephalopathy-immune reconstitution inflammatory syndrome. Brain. 2011;134:2687–2702. doi: 10.1093/brain/awr206. [DOI] [PubMed] [Google Scholar]
- 12.Metz I, Radue EW, Oterino A, et al. Pathology of immune reconstitution inflammatory syndrome in multiple sclerosis with natalizumab-associated progressive multifocal leukoencephalopathy. Acta Neuropathol. 2012;123:235–245. doi: 10.1007/s00401-011-0900-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahlhaus S, Hoepner R, Chan A, et al. Disease course and outcome of 15 monocentrically treated natalizumab-associated progressive multifocal leukoencephalopathy patients. J Neuro Neurosurg Psychiatry. 2013;84:1068–1074. doi: 10.1136/jnnp-2013-304897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryschkewitsch CF, Jensen PN, Monaco MC, Major EO. JC virus persistence following progressive multifocal leukoencephalopathy in multiple sclerosis patients treated with natalizumab. Ann Neuro. 2010;68:384–391. doi: 10.1002/ana.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine AJ, Sorbello A, Kortepeter C, Scarazzini L. Progressive multifocal leukoencephalopathy after natalizumab discontinuation. Ann Neurol. 2014;75:108–115. doi: 10.1002/ana.24051. [DOI] [PubMed] [Google Scholar]
- 16.Sindic CJ, Trebst C, Van Antwerpen MP, et al. Detection of CSF-specific oligoclonal antibodies to recombinant JC virus VP1 in patients with progressive multifocal leukoencephalopathy. J Neuroimmunol. 1997;76:100–104. doi: 10.1016/s0165-5728(97)00037-4. [DOI] [PubMed] [Google Scholar]
- 17.Weber T, Trebst C, Frye S, et al. Analysis of the systemic and intrathecal humoral immune response in progressive multifoca eukoencephalopathy. J Infect Dis. 1997;176:250–254. doi: 10.1086/514032. [DOI] [PubMed] [Google Scholar]
- 18.Warnke C, Mausberg AK, Stettner M, et al. Natalizumab affects the T-cell receptor repertoire in patients with multiple sclerosis. Neurology. 2013;81:1400–1408. doi: 10.1212/WNL.0b013e3182a84101. [DOI] [PubMed] [Google Scholar]
- 19.Warnke C, Ramanujam R, Plavina T, et al. Changes to anti-JCV antibody levels in a Swedish national MS cohort. J Neurol Neurosurg Psychiatry. 2013;84:1199–1205. doi: 10.1136/jnnp-2012-304332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piehl F, Holmen C, Hillert J, Olsson T. Swedish natalizumab [Tysabri) multiple sclerosis surveillance study. Neurol Sci. 2011;31(suppl 3):289–293. doi: 10.1007/s10072-010-0345-y. [DOI] [PubMed] [Google Scholar]
- 21.Sehr P, Zumbach K, Pawlita M. A generic capture ELISA for recombinant proteins fused to glutathione S-transferase: validation for HPV serology. J Immunol Methods. 2001;253:153–162. doi: 10.1016/s0022-1759(01)00376-3. [DOI] [PubMed] [Google Scholar]
- 22.Warnke C, Pawlita M, Dehmel T, et al. An assay to quantify species-specific anti-JC virus antibody levels in MS patients. Mult Scler. 2013;19:1137–1144. doi: 10.1177/1352458513475489. [DOI] [PubMed] [Google Scholar]
- 23.Reiber H, Peter JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci. 2001;184:101–122. doi: 10.1016/s0022-510x(00)00501-3. [DOI] [PubMed] [Google Scholar]
- 24.Ryschkewitsch C, Jensen P, Hou J, et al. Comparison of PCR-Southern hybridization and quantitative real-time PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. J Virol Methods. 2004;121:217–221. doi: 10.1016/j.jviromet.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 25.Warnke C, Adams O, Kieseier BC. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N Engl J Med. 2009;361:2489. author reply 2490. [PubMed] [Google Scholar]
- 26.Warnke C, Smolianov V, Dehmel T, et al. CD34+ progenitor cells mobilized by natalizumab are not a relevant reservoir for JC virus. Mult Scler. 2011;17:151–156. doi: 10.1177/1352458510385834. [DOI] [PubMed] [Google Scholar]
- 27.Subramanyam M, Plavina T, Khatri BO, et al. The effect of plasma exchange on serum anti-JC virus antibodies. Mult Scler. 2013;19:912–919. doi: 10.1177/1352458512467502. [DOI] [PubMed] [Google Scholar]
- 28.Ayzenberg I, Lukas C, Trampe N, et al. Value of MRI as a surrogate marker for PML in natalizumab long-term therapy. J Neuro. 2012;259:1732–1733. doi: 10.1007/s00415-012-6426-5. [DOI] [PubMed] [Google Scholar]
- 29.Wattjes MP, Verhoeff L, Zentjens W, et al. Punctate lesion pattern suggestive of perivascular inflammation in acute natalizumab-associated progressive multifocal leukoencephalopathy: productive JC virus infection or preclinical PML-IRIS manifestation? J Neurol Neurosurg Psychiatry. 2013;84:1176–1177. doi: 10.1136/jnnp-2013-304986. [DOI] [PubMed] [Google Scholar]
- 30.Vennegoor A, Wattjes MP, van Munster ET, et al. Indolent course of progressive multifocal leukoencephalopathy during natalizumab treatment in MS. Neurology. 2011;76:574–576. doi: 10.1212/WNL.0b013e31820b7644. [DOI] [PubMed] [Google Scholar]
- 31.Tan CS, Chen Y, Viscidi RP, et al. Discrepant findings in immune responses to JC virus in patients receiving natalizumab. Lancet Neurol. 2010;9:565–566. doi: 10.1016/S1474-4422(10)70124-1. author reply 566–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Bord E, Tompkins T, et al. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N Engl J Med. 2009;361:1067–1074. doi: 10.1056/NEJMoa0904267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jilek S, Jaquiery E, Hirsch HH, et al. Immune responses to JC virus in patients with multiple sclerosis treated with natalizumab: a cross-sectional and longitudinal study. Lancet Neurol. 2010;9:264–272. doi: 10.1016/S1474-4422(10)70006-5. [DOI] [PubMed] [Google Scholar]
- 34.Saure C, Warnke C, Zohren F, et al. Natalizumab and impedance of the homing of CD34+ hematopoietic progenitors. Arch Neuro. 2011;68:1428–1431. doi: 10.1001/archneurol.2011.238. [DOI] [PubMed] [Google Scholar]
- 35.Warnke C, Adams O, Hartung HP, Kieseier BC. Assessment of JC virus DNA in blood and urine from natalizumab-treated patients. Ann Neurol. 2011;69:215–216. doi: 10.1002/ana.22305. author reply 216. [DOI] [PubMed] [Google Scholar]
- 36.Schwab N, Schneider-Hohendorf T, Posevitz V, et al. L-Selectin is a possible biomarker for individual PML risk in natalizumab-treated MS patients. Neurology. 2013;81:865–871. doi: 10.1212/WNL.0b013e3182a351fb. [DOI] [PubMed] [Google Scholar]
- 37.Trampe AK, Hemmelmann C, Stroet A, et al. Anti-JC virus antibodies in a large German natalizumab-treated multiple sclerosis cohort. Neurology. 2012;78:1736–1742. doi: 10.1212/WNL.0b013e3182583022. [DOI] [PubMed] [Google Scholar]
- 38.Plavina T, Subramanyam M, Bloomgren G, et al. Anti-JCV antibody index further defines PML risk in natalizumab-treated MS patients; Paper presented at: 27th Annual Meeting of the Consortium of Multiple Sclerosis Centers; May 29-Jun 1, 2013; Orlando, FL. [Accessed February 8, 2014]. Available at: https://cmscactrims.confex.com/cmscactrims/2013/webprogram/Paper1642.html. [Google Scholar]
- 39.Wattjes MP, Richert ND, Killestein J, et al. The chameleon of neuroinflammation: magnetic resonance imaging characteristics of natalizumab-associated progressive multifocal leukoencephalopathy. Mult Scler. 2013;19:1826–1840. doi: 10.1177/1352458513510224. [DOI] [PubMed] [Google Scholar]
- 40.Giudici B, Vaz B, Bossolasco S, et al. Highly active antiretroviral therapy and progressive multifocal leukoencephalopathy: effects on cerebrospinal fluid markers of JC virus replication and immune response. Clin Infect Dis. 2000;30:95–99. doi: 10.1086/313598. [DOI] [PubMed] [Google Scholar]