Figure 1.
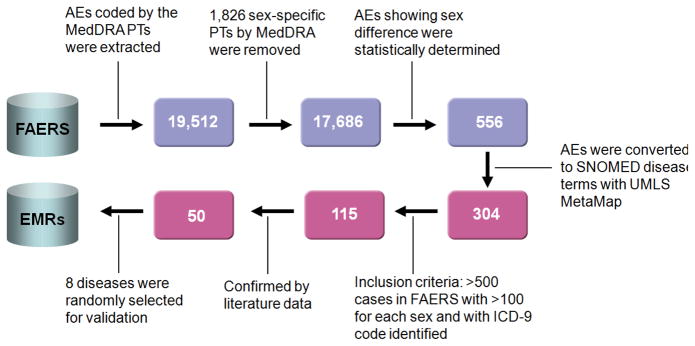
Flowchart of study on FDA Adverse Event Reporting System (FAERS) for disease monitoring. Blue boxes show the number of AEs (top row) and red boxes (bottom row) show the number of diseases.

Flowchart of study on FDA Adverse Event Reporting System (FAERS) for disease monitoring. Blue boxes show the number of AEs (top row) and red boxes (bottom row) show the number of diseases.