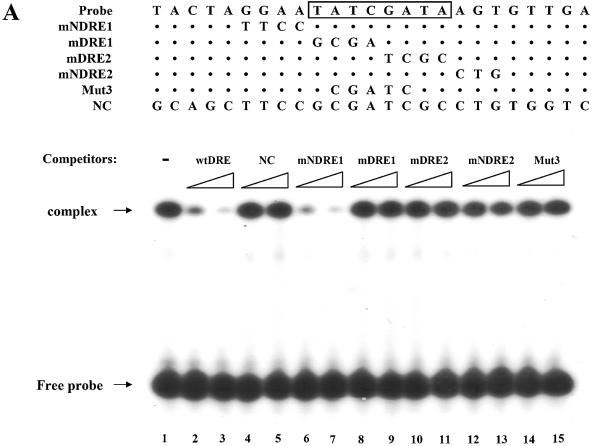
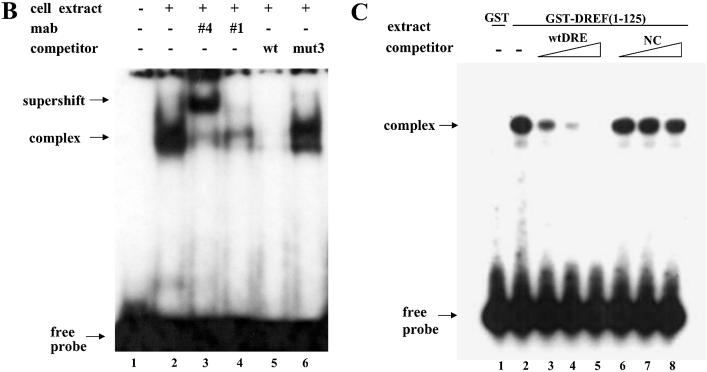
Figure 5.
Identification of DREF as a protein factor that binds to dsps2 promoter. (A) EMSA of DRE-containing sequence in SL2 cell extracts. Sequences of probe or competitor DNAs are shown in the upper portion of the figure, the competitor DNA fragments in increasing amounts in the center portion and the results of the competition experiments in the lower portion. The DRE consensus sequence is shown in the box. In the mutant competitors, only mutated sequences are indicated and the sequences that are the same as wild type are marked as dots. NC designates non-specific competitor. (B) Supershift of retarded band by anti-DREF antibody. 32P-labeled probes were incubated with SL2 nuclear extracts in the absence of antibody (lanes 2, 5 and 6) or in the presence of anti-DREF monoclonal antibody (lanes 3 and 4). The extracts were also incubated with an excess amount of unlabeled wild-type oligonucleotide as specific competitor (lane 5) or mutant3 oligonucleotide (see Materials and Methods) as non-specific competitor (lane 6). The probe was also incubated without extract, antibody and competitor (lane 1). (C) EMSA with GST-DREF fusion protein. SL2 nuclear extracts were incubated with GST protein (lane 1) or with GST-DREF (1–125) in the absence (lane 2) or presence of excess amounts of specific competitor (wtDRE, lanes 3–5) or non-specific competitor (NC, lanes 6–8) as shown at the top of the panel. Concentrations of competitors used are given in Materials and Methods.