Abstract
The Medicare Program is conducting a randomized trial of care management services among fee-for-service (FFS) beneficiaries called the Medicare Health Support (MHS) pilot program. Eight disease management (DM) companies have contracted with CMS to improve clinical quality, increase beneficiary and provider satisfaction, and achieve targeted savings for chronically ill Medicare FFS beneficiaries. In this article, we present 6-month intervention results on beneficiary selection and participation rates, mortality rates, trends in hospitalizations, and success in achieving Medicare cost savings. Results to date indicate limited success in achieving Medicare cost savings or reducing acute care utilization.
Introduction
Background
In 2001, an Institute of Medicine (2001) report highlighted the many discrepancies between what is known about effective treatments for chronic diseases and the care that actually is received by the majority of Americans. Even when best practices have been established, good chronic care is intrinsically harder to achieve than good acute care (Vladeck, 2001), and no single model has emerged as the best means for organizing care management for persons with chronic illnesses. Good chronic care requires methods for providing ongoing resources and supports for patients' self-management behaviors and skills. It also requires adopting best practices from evolving evidence-based guidelines and collaboration among medical providers, non-medical providers, and payers.
Medicare beneficiaries with multiple progressive chronic diseases are a large and costly subgroup of the Medicare population. These persons must navigate a system that has been structured and financed to manage acute, rather than long-term, health problems. When older beneficiaries seek care, they are typically treated in discrete settings rather than being managed holistically (Todd and Nash, 2001; Anderson, 2002). Consequently, many patients have difficulty integrating what has been prescribed into their daily routines and frequently receive conflicting advice. Their providers often lack timely or complete information to fully assess their needs and prevent acute exacerbations of their chronic illnesses. Gaps often emerge between what is appropriate care for chronic conditions and the care actually received (McGlynn et al., 2003; Jencks, Huff, and Cuerdon, 2003).
Controlling costs by managing the care of the chronically ill is receiving increasing attention from both researchers and policymakers (Institute of Medicine, 2001; Crippen, 2002; Goetzel et al., 2002, 2003; Bodenheimer, Wagner, and Grumbach, 2002; Foote, 2003; Disease Management Association of America, 2005; Thorpe and Howard, 2006). The literature on the effect of DM in controlling costs is growing (Heany and Goetzel, 1997; Norris et al., 2002; U.S. Congressional Budget Office, 2004; Knight et al., 2005; Goetzel et al., 2005; Mattke, Seid, and Ma, 2007). Cost-benefit studies typically calculate a return-on-investment (ROI) index: i.e., the amount of money saved for every dollar spent. An ROI of 1.0 reflects a cost-neutral program. The range of ROI ratios in DM programs for heart failure (HF) is wide, ranging from −2.74 to 14.18 (Goetzel et al., 2005). Returns to managing multiple chronic illnesses ranged from 4.4 to 10.9 (Goetzel et al., 2005). Several studies show specific gains in improving the quality of care in terms of fewer HF inpatient days (Rich et al., 1995) and reductions in diabetic HbA1c levels (Wagner et al., 2001; U.S. Congressional Budget Office, 2004; Knight et al., 2005).
Many researchers have questioned these early results on methodological grounds (Short, Mays, and Mittler, 2003; Smith et al., 2005; Goetzel et al., 2005). After an extensive review of the literature, the U.S. Congressional Budget Office (2004), based on earlier studies, concluded the following:
“Although a few programs showed some cost reductions, these findings were often modest and inconsistent. Both a smaller number and [smaller] percentage of studies showed reductions in costs than [showed] improvements in quality of care… Thus, al though DM may improve the quality of care for beneficiaries with chronic disease, long-term studies may be required to show the economic benefit and financial return on investment.”
Congress designed the MHS pilot to address perceived current failings of the health care system for chronically ill, Medicare FFS beneficiaries and to allow for a large-scale, randomized evaluation of DM's ability to improve quality of care and reduce health care costs (Medicare Prescription Drug, Improvement, and Modernization Act, 2003). Eight private DM companies were selected for a 3-year MHS Phase I pilot. The overall design of the MHS pilot follows an intent-to-treat prerandomization model. As required in the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 legislation, section 1807(f)(1)(B)(ii), Medicare health support organizations (MHSOs) had to demonstrate that they were capable of “assum[ing] financial risk” to assure that they were financially capable of serving as risk-bearing entities. CMS invoked risk taking by requiring MHSOs to achieve at least 5 percent gross savings on Medicare claims costs (rescinded later during pilot) or return all of the management fees they had received. MHSOs agreed to both budget neutrality (i.e., covering fees through savings) and the additional 5 percent requirement in their contract terms and conditions that made it harder to achieve overall financial savings. The MHSOs are also at financial risk for improvement in beneficiary satisfaction and clinical quality of care. If these programs or components thereof are successful, the MHS pilot may be expanded to a Phase II, which may include national implementation.
Purpose
In this article, we provide a detailed overview of MHS's experimental design, followed by various analyses of program performance through the first 6 months of operation. Other analyses will be conducted at months 18 and 36. We describe the MHS pilot, emphasizing the financial requirements, discuss the data and methods used in this initial analysis, and finally, report the results focusing on trends in hospitalizations and success in achieving Medicare cost savings.
Methods
Description and Evaluation of the MHS
The MHS pilot programs target beneficiaries with HF or diabetes mellitus with significant comorbidities—two prevalent and costly chronic conditions within the Medicare FFS population. Beneficiaries in FFS have a myriad other chronic conditions, however. Consequently, all programs have implemented a holistic approach to care management that addresses beneficiary needs, regardless of the associated condition. The MHS programs incorporate features from private-sector DM and case management programs. Strategies to increase quality of care and decrease costs include educating beneficiaries about their conditions, improving communication with providers, and improving self-management skills. Successfully changing beneficiary behavior should result in a beneficiary's ability to better manage their chronic conditions, a slower rate in functional decline, and fewer acute exacerbations leading to costly hospitalizations. Reducing major cost drivers, such as acute care utilization, is necessary to reduce overall program costs.
Selection, Eligibility, and Randomization
Beneficiaries were eligible for randomization into the MHS if they were enrolled in Medicare FFS, had a claims-based indication of HF or DM, and had a hierarchical condition categories (HCC) risk score of 1.35 or greater1. Beneficiaries were excluded if they did not have Medicare as their primary payer or were enrolled in the Medicare end-stage renal disease program, hospice, or another CMS-sponsored FFS chronic care demonstration. CMS randomly assigned roughly 20,000 beneficiaries into an intervention group and another 10,000 into a comparison group for each MHSO. The general approach used stratified randomization to ensure equal distribution between intervention and comparison groups of persons with the following characteristics: a claims-based diagnosis of HF or DM, three levels of HCC risk scores, and Medicaid enrollment. Randomization occurred on May 11, 2005. Eligibility for MHS is lost for periods when beneficiaries (1) met any of the previously mentioned exclusion criteria, (2) joined a Medicare managed care plan, (3) failed to pay their Medicare premium for physician services, (4) died, (5) entered the end-stage renal disease or hospice program, or (6) Medicare became their secondary payer. Beneficiaries could return to MHS by regaining eligibility.
Selection of MHSOs and Their Interventions
CMS is supporting programs in eight geographic areas where at least 10 percent of the Medicare population is in FFS (Table 1). Only one pilot program was selected per geographic area. The target areas are in different market areas east of the Mississippi River or in Oklahoma. Several programs serve urban and suburban populations; others target metropolitan and rural communities. There are significant minority populations of African-American, American Indian, and Hispanic beneficiaries among the populations served.
Table 1. Medicare Health Support Organizations (MHSOs) Pilot Programs, by Target Area and Launch Date.
| MHSO | Target Area | Launch Date |
|---|---|---|
| Healthways | Maryland and District of Columbia | August 1, 2005 |
| LifeMasters Supported SelfCare | Oklahoma | August 1, 2005 |
| Health Dialog Services Corporation | Pennsylvania (Western Region) | August 15, 2005 |
| McKesson Health Solutions, LLC | Mississippi | August 22, 2005 |
| Aetna Life Insurance Company | Chicago, IL (Surrounding Area) | September 1, 2005 |
| Cigna Health Support | Georgia (Northern Region) | September 12, 2005 |
| Green Ribbon Health | Florida (West-Central Region) | November 1, 2005 |
| XLHealth Corporation | Tennessee (Selected Counties) | January 16, 2006 |
NOTE: These eight geographic areas are where at least 10 percent of the Medicare population is in fee-for-service.
SOURCE: Centers for Medicare & Medicare Services: Data from unpublished reports submitted by Medicare health support organizations, 2005.
The eight MHSOs launched their programs between August 1, 2005, and January 16, 2006. Program start dates occurred 2.5 to 8 months after the randomization date. This hiatus resulted in minor differences between the intervention and comparison populations when organizations began to engage beneficiaries, as shown later.
Intent-to-Treat and Prerandomization Study Design
While CMS had implemented enrollment-or participation-based payment schemes as part of prior DM demonstrations, this was the first initiative to include intent-to-treat and prerandomization design features. The performance conditions do not include participation requirements; rather, MHSOs are held accountable for all beneficiaries in their intervention groups, regardless of whether they actually agreed to participate.
Beneficiary Outreach Period
The MHSOs' programs began with a 6-month outreach period, during which they were expected to contact beneficiaries, gain their verbal consent, and begin intervention services. In their applications and contracts with CMS, MHSOs predicted they would achieve budget neutrality or better within 12 months of the pilot's start. This implied a rapid expected start up in care management activities along with engaging (signing up) beneficiaries. We used each beneficiary's date of engagement to calculate average time from MHSO start date to engagement. The MHSOs had a strong incentive to recruit all beneficiaries because they were at risk for the full intervention group and because they received fees for all beneficiaries assigned to the intervention group except for those who were ineligible or expressly declined participation. At the end of the outreach period, MHS monthly payments (i.e., fees) ceased for any non-participating beneficiary.
Analytic Methods
The experimental design within this pilot calls for a pre/post intervention/comparison analytic approach—sometimes referred to as a difference-in-differences approach. We used this strategy to construct estimates of all performance outcomes of each pilot program. Estimation techniques differ depending on the nature of the performance variable. For each MHSO, we evaluated the 6-month intervention period relative to a 12-month baseline period prior to the start of its program. We included only beneficiaries who were alive at the start date of each MHSO and accounted for differential periods of eligibility by weighting beneficiaries proportionate to their eligible days.
Randomization Equality and Intervention Participation
We define a participant as an eligible beneficiary who verbally consented to participate in an MHS program and actually participated for at least 1 day. Non-participants are individuals in the intervention group who were unreachable or refused to participate. We tested for differences in demographic, clinical, and utilization characteristics between the intervention and comparison groups in the year prior to the start of each pilot to determine whether differences emerged between the intervention and comparison groups due to the lag between the randomization and the start dates. We tabulated the percentage of beneficiaries by three disease status groups: (1) HF only, (2) diabetes mellitus-only, and (3) HF with diabetes mellitus. We also estimated mean HCC and Charlson Index scores reflecting beneficiary clinical severity. The Charlson Index is a weighted average of the presence of several comorbid conditions. The percentage of beneficiaries with Medicaid enrollment was also estimated, another severity proxy. To better understand cost differences across MHSOs, hospitalization and emergency room rates were tabulated. We tested for differences in average historical per beneficiary per month (PBPM) Medicare payments between the intervention and comparison groups at randomization and at the start of the pilot.
To test for the relative differences, or selection bias, in characteristics between participants and non-participants, we estimated logistic models of participation among intervention beneficiaries. Variables tested in the model included prior year costs, sex, age, urban residence, HCC scores, disease category (HF or HF and diabetes mellitus), Medicaid enrollment, and death during 1 of 6 pilot months.
Quality of Care
We report on changes in rates of acute hospitalizations (all-cause, HF-only, and diabetes mellitus-only hospitalizations), as they can reflect the quality of outpatient care; rates are constructed for the first 6-month intervention period and, to remove the seasonality influence on these measures, for a comparable 6-month period during the year prior to each MHSO's go-live date. We fit a log-linear regression model with robust variance estimation to adjust for any repeated (pre- and post-) hospitalizations or emergent visits.
Financial Analyses
MSHOs were paid monthly fees of $74 to $159 (or $888 to $1,908 annually) for every eligible intervention beneficiary except those who declined participation during the first 6-month (enrollment) period. After that, MHSOs received fees for participating beneficiaries only. For an MHSO to keep its fees, Medicare health care outlays for the intervention group (PBPMI) were to be at least 5 percent less than the outlays for the comparison group (PBPMC) less an additional amount equal to the fees. Although CMS eventually rescinded the 5 percent minimum requirement, we use it as a measure of financial success in this article because it still was in force during the first 6 months. The potential amount of refund (RF) of fees is expressed using the following maxi-min algorithm:
| (1) |
where
RS = the required 5 percent net Medicare savings (in dollar terms),
GS = gross Medicare savings,
MF = the monthly management fee, and
RS – GS = net required savings.
The maxi-min refund algorithm produces three payment regimes depending on the organization's success in reducing costs:
refund all fees if: (PBPMI / PBPMC) > 0.95,
partial fee refund if: (0.95 – MF / PBPMC) < (PBPMI / PBPMC) < 0.95, or
no fee refund if: (PBPMI / PBPMC) < (0.95–MF / PBPMC).
If intervention PBPM costs are not reduced by at least 5 percent under the original contract terms, an MHSO must repay all fees it received. Medicare savings that are less than 5 percent do not offset any of the MHSOs' refund obligations. Conversely, if the MHSO reduces PBPMs by 5 percent plus the fee, then it keeps all of its fees. Given the congressional concern over paying MHSOs upfront fees eventually amounting to a few hundred million dollars, CMS agreements with MHSOs required them to achieve at least budget neutrality through the pilot's first 12 months. Gross savings to Medicare was calculated based on a difference-in-differences in PBPM growth rates. This produces the best estimate of short-run success controlling for any differences in PBPMs at the beneficiary (and MHSO) level. Any differences in average base year PBPMs between intervention and comparison groups are factored out by using beneficiary-specific growth rates. When conducting pairwise tests of beneficiary changes in PBPMs between the base and pilot periods, we weighted changes by the beneficiary's fraction of eligible days during the 6-month pilot period. This avoids overstating the changes for beneficiaries exposed to the intervention for a shorter period.
Data
We used five types of data for the analyses: (1) the enrollment data base to obtain demographic characteristics and date of death information, (2) a participant status file to determine the participation decision and engagement dates of the intervention beneficiaries, (3) an all-qualified file to identify the disease groups to which each beneficiary was assigned via randomization, (4) a daily eligibility file to identify beneficiaries as eligible or ineligible for each day of the intervention period, and (5) our own development of Medicare Parts A and B claims data for 2004-2006 to capture service utilization and Medicare payments.
Only claims for services that occurred during periods of eligibility were included in the utilization and payment measures. For the 12-month prepilot period, we included claims if services commenced during any month that the beneficiary had Medicare FFS. For the pilot period, we included claims if services commenced on any day that the beneficiary met the MHS eligibility criteria. Future analyses will use the claims database developed by the financial reconciliator with longer run-out periods and deleting claims the day (instead of month) a beneficiary becomes ineligible.
Results
Participation
Reported MHSO participation rates during the first 6 months of the pilot programs are shown in Table 2. To maintain confidentiality, MHSOs in this and subsequent tables and figures are identified only by a number that does not necessarily correspond with the order that they appear in Table 1. Participation rates ranged from a high of 93 percent to a low of 65 percent. Mean time-to-recruitment ranged from 38 to 100 days across the MHSOs (not shown); consequently, our preliminary evaluation reflects results of intervention activities for only 2.8 to 4.8 months on average. While results at 6 months may underestimate eventual intervention effects, a fast start-up will be necessary to achieve the financial benchmark of budget neutrality within the first year and a 5-percent savings within the 3-year pilot window.
Table 2. Pilot Participating Rates During the First 6 Months, by Medicare Health Support Organizations (MHSOs).
| MHSO | Participation Rate |
|---|---|
|
| |
| Percent | |
| 1 | 83.6 |
| 2 | 93.2 |
| 3 | 65.0 |
| 4 | 75.6 |
| 5 | 80.3 |
| 6 | 83.2 |
| 7 | 82.6 |
| 8 | 70.0 |
SOURCE: Centers for Medicare & Medicare Services: Data from unpublished reports submitted by Medicare health support organizations, 2005.
Characteristics
Table 3 characterizes the beneficiaries in the intervention group looking back 1 year from the start of the pilot. We found few statistically significant differences between the characteristics of intervention and comparison beneficiaries, implying that randomization held until the start of the pilot (McCall et al., 2007). Although few differences were found between the intervention and comparison groups for the eight MHSOs, it is possible that unobserved differences in their beneficiary mix may affect their likelihood of achieving the pilot's required savings targets.
Table 3. Characteristics of Intervention Beneficiaries in Medicare Health Support Organization (MHSO) Pilot Program Base Year1.
| MHSO | Condition | HCC Score | Medicaid (Percent) | Charlson Index | Hospitalization Rate per 100 | Emergency Room Visits per 100 | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| HF Only | Diabetes Only | HF and Diabetes | Mean | %>3.10 | |||||
|
| |||||||||
| Percent | |||||||||
| 1 | 24 | 55 | 20 | 2.5 | 25 | 14 | 4.0 | 73 | 55 |
| 2 | 22 | 55 | 22 | 2.6 | 26 | 17 | 4.1 | 98 | 69* |
| 3 | 20 | 58 | 22 | 2.4 | 21 | 34 | 3.6 | 76 | 84 |
| 4 | 21 | 54 | 25 | 2.3 | 19 | 43 | 3.6 | 90 | 103 |
| 5 | 26 | 51 | 23 | 2.5 | 24 | 16 | 3.9 | 104* | 51 |
| 6 | 19 | 60 | 21 | 2.4 | 21 | 25 | 3.7* | 79*** | 87 |
| 7 | 20 | 60 | 20 | 2.5 | 25 | 16 | 4.0** | 86 | 59 |
| 8 | 26 | 49 | 25 | 2.4 | 21 | 21 | 3.7 | 91 | 78 |
| Average | 22 | 55 | 22 | 2.5 | 24 | 23 | 3.8 | 86 | 72 |
p<0.10.
p<0.05.
p<0.01.
Base year is 12 months prior to MHSO start date. Includes only beneficiaries living at the time of the start date. Rates are weighted by months of Medicare Parts A and B fee-for-service eligibility.
NOTES: t-tests of differences between intervention and comparison groups. HCC is hierarchical condition categories. HF is heart failure. Numbers in column 1 refer to MHSO pilot program.
SOURCES: Centers for Medicare & Medicaid Services: Data from Medicare Inpatient, Outpatient, and Physician/Supplier Claims, 2004-2006; Medicare Enrollment Data Base File.
Our logistic regression model predicting participation in the programs (not shown) revealed that many beneficiary characteristics differed between participants and non-participants. The likelihood of participation was lower if prior year costs were high or if the beneficiary died early on in the 6-month pilot period. Holding prior year's PBPM and death rate constant, participants were more likely than non-participants to be female, younger, have diabetes-only, to be in better health (lower HCC scores), and less likely to have qualified for Medicare due to a disability (age<65 years) or be enrolled in Medicaid.
Difference in PBPMs
Table 4 displays the percentage difference in average Medicare payments for the 12 months prior to randomization and the 12 months prior to each MHSO's start date. Positive percentages imply higher intervention PBPMs. No statistically significant differences in PBPMs were found between the intervention and comparison groups at the time of randomization. By the start dates, seven of eight MHSOs began with intervention beneficiaries who were 1-6 percent more costly than their comparison group. Only one difference, however, was statistically significant. Small deviations in PBPMs from randomization to start date are to be expected given the substantial volatility in beneficiary average monthly costs and minor changes in beneficiary mix. Nonetheless, CMS eventually agreed to adjust any differences in intervention and comparison group PBPMs for any differences due to the drift in average base year PBPMs prior to the start date. Any differences, however, will not effect our calculations of growth rates that automatically adjust for any base year differences in intervention and comparison PBPMs.
Table 4. Percentage Difference in Intervention and Comparison Per Beneficiary Per Month (PBPM) Costs at Randomization and Start Date for Medicare Health Support Organizations (MHSOs).
| MHSO | Randomization1 | Start Date2 |
|---|---|---|
|
| ||
| Percent | ||
| 1 | 1.2 | 1.0 |
| 2 | -1.2 | 3.0 |
| 3 | -2.6 | 1.3 |
| 4 | -0.7 | 2.2 |
| 5 | 0.8 | 3.1 |
| 6 | -1.0 | 6.1*** |
| 7 | 0.1 | -1.1 |
| 8 | 0.0 | 0.2 |
*** p<0.01.
PBPM Medicare payments weighted by months of eligibility for Medicare Part A in 12 months prior to randomization.
Medicare payments weighted by fraction of eligible days in pilot period.
SOURCE: Centers for Medicare & Medicaid Services: Data from Medicare Inpatient, Outpatient, and Physician/Supplier Claims, 2004-2006.
Levels and Trends in Hospitalizations and Mortality
Hospitalization Rates
Table 5 presents ratios of base period to 6-month hospitalization rates for HF-only and diabetes mellitus-only beneficiaries for the intervention and comparison groups. Base periods are the 6-month periods in the prior year that correspond to the 6-month pilot periods to control for variations in seasonal utilization. Table 5 shows that non-HF admissions are far more numerous than HF-related admissions for HF-only beneficiaries. Among HF beneficiaries, the odds of a non-HF admission ranged from 2.5 (MHSO 2) to 5.0 (MHSO 3). Table 5 shows that diabetes mellitus-only beneficiaries were far more likely to have a non-DM admission. Thus, MHSOs will have to reduce hospitalizations for a multiplicity of clinical conditions other than HF and DM to achieve cost savings.
Table 5. Ratio of 6-Month Medicare Health Support Organization (MHSO) Pilot Programs to Base Period Hospitalization Rates, Heart Failure (HF)-Only and Diabetes-Only Beneficiaries, by Intervention and Comparison Groups.
| MHSO | HF–Only Beneficiaries | Diabetes-Only Beneficiaries | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Intervention Group | Comparison Group | Intervention Group | Comparison Group | |||||||
|
|
|
|
|
|||||||
| All Hosp1 | HF Hosp2 | NHF/HF3 | All Hosp1 | HF Hosp2 | All Hosp1 | Diabetes Hosp4 | NDIAB/Diabetes5 | All Hosp1 | Diabetes Hosp5 | |
| 1 | 1.02 | 0.80 | 3.89 | 0.95 | 0.72 | 1.09 | 0.92 | 31.88 | 1.17 | 1.00 |
| 2 | 0.90*** | 0.66*** | 2.49 | 0.77*** | 0.59*** | 1.04 | 0.91 | 66.80 | 1.02 | 1.00 |
| 3 | 1.00 | 1.01 | 5.03 | 1.12 | 1.05 | 1.19 | 1.19 | 54.40 | 1.17 | 0.95 |
| 4 | 0.90*** | 0.68*** | 3.01 | 0.91* | 0.73*** | 1.03 | 0.93 | 65.00 | 0.96 | 0.56*** |
| 5 | 0.87*** | 0.62*** | 2.82 | 0.87*** | 0.69*** | 1.00 | 0.97 | 12.66 | 1.06 | 0.72** |
| 6 | 0.90*** | 0.68*** | 2.70 | 0.93 | 0.72*** | 1.01 | 0.67*** | 12.57 | 0.98 | 0.90 |
| 7 | 0.89*** | 0.64*** | 3.11 | 0.90* | 0.55*** | 1.06** | 1.05 | 15.10 | 1.07 | 1.00 |
| 8 | 0.87*** | 0.55*** | 3.41 | 0.83*** | 0.62*** | 1.02 | 0.82* | 109.00 | 0.97 | 0.90 |
p<0.10.
p<0.05.
p<0.01.
All hosp is ratio of the 6-month pilot to the base period for all hospitalization rates.
HF hosp is ratio of the 6-month pilot to the base period for hospitalization rates for HF.
NHF/HF is ratio of non-HF hospitalization rates to HF hospitalization rates.
Diabetes hosp is ratio of the 6-month pilot to the base period hospitalization rates for diabetes.
NDIAB/Diabetes is ratio of non-diabetes hospitalization rates to diabetes hospitalization rates.
NOTES: t-tests of pilot versus base period differences. Base period covers same 6 months as MHSO pilot period. HOSP is hospitalization rate. NDIAB is non-diabetes. NHF is non-heart failure.
SOURCE: Centers for Medicare & Medicaid Services: Data from Medicare Inpatient, Outpatient, and Physician/Supplier Claims, 2004-2006.
Table 5 indicates that all-cause hospitalization rates for HF-only beneficiaries during the 6-month intervention period were stable or declining compared to a 6-month baseline period. The rates for HF-related admissions for HF-only beneficiaries were down sometimes by one-third or more; however, the same trends are true for the comparison group. The only statistically significant difference-in-differences is for MHSO 2, where its comparison group all-cause hospitalization rate declined more in the intervention period than its intervention group (0.77 versus 0.90; p<0.05, not shown). Trends in all-cause hospitalizations for intervention diabetes mellitus-only beneficiaries were rising, but only one MHSO's trend was significant. Trends for diabetes mellitus-related admissions generally fell, although, again, only one trend was statistically significant comparison group trends in all-cause and diabetes mellitus-related admissions were similar to intervention trends.
Levels and Trends in Medicare Outlays
PBPM Growth Rates
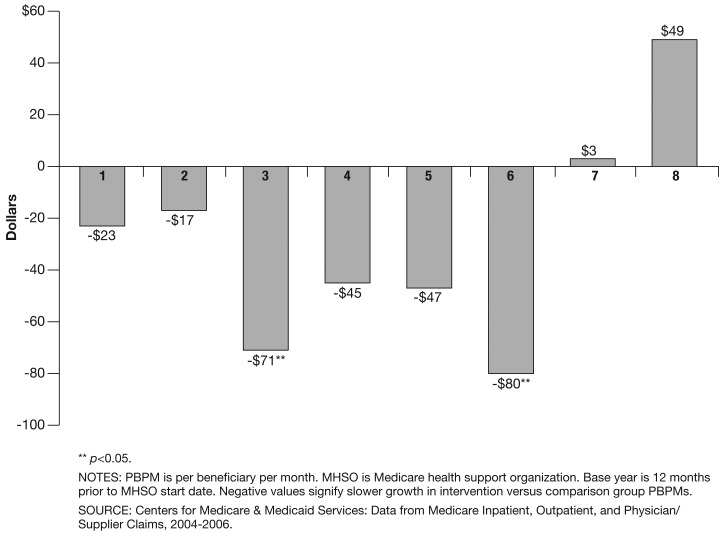
In Figure 1, we present a difference-in-differences analysis of trends in PBPMs for each of the eight MHSOs starting from each beneficiary's own base year PBPM. MHSO 1's intervention group PBPM increased by $208 between the baseline year and the first 6-month period. Over the same period, MHSO 1's comparison group PBPM increased $231, implying $23 in PBPM Medicare gross savings. Six of the eight MHSOs exhibited lower relative rates of growth in Medicare PBPM payments between the year prior and first 6 months of the pilot program. Yet, only the savings for MHSO 3 and 6 were statistically significant from zero (p<0.05). To achieve statistical significance, the differences-in-trends needed to be between $62-$90, or 4.4-5.8 percent of the comparison group's PBPM. MHSO 7's intervention PBPM paralleled its comparison group, while MHSO 8's intervention PBPM grew faster than its comparison group's PBPM.
Figure 1. Difference in Changes in Medicare PBPM Between Intervention and Comparison Groups From Base Year Through First 6 Pilot Months, by MHSO.
Net Savings Through 6 Months
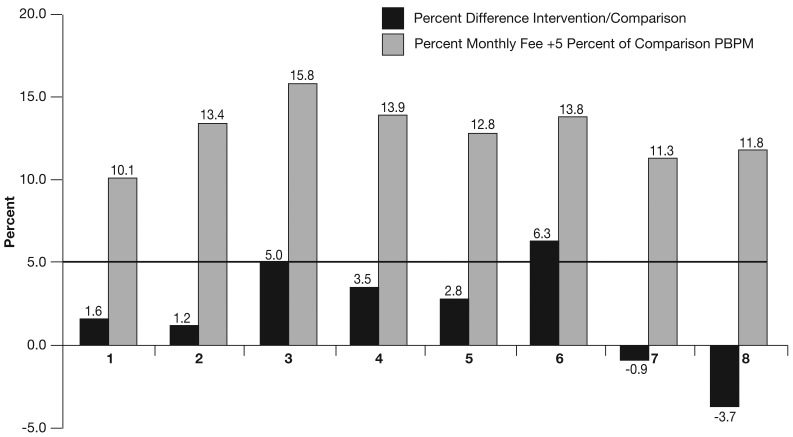
Figure 2, based on our method, shows each MHSO's early success in meeting the original pilot's financial requirement of 5 percent net savings over a 3-year period. (Actual refunds will be based on formula [1]). Figure 2's light bars show the percentage that the MHSO had to reduce the intervention's PBPM relative to its comparison group's PBPM to keep all its management fees. Monthly management fees during the first 6 months, as a proportion of comparison group PBPMs, ranged from a low of 5.3 percent (MHSO 1) to a high of 11.2 percent (MHSO 3). For example, MHSO 1's fee was 5.1 percent of its comparison group's average monthly health care payments. Adding the required 5 percent savings meant that MHSO 1 needed to save a total of 10.1 percent to keep all fees. PBPM reductions to keep all fees ranged from a low of 10.1 percent (MHSO 1) to a high of 15.8 percent (MHSO 3).
Figure 2. Proportion of Net Total Savings1 Required and Achieved Through Pilot's First 6 Months, by MHSO.
1 Percentages on top of light bars include required 5 percent Medicare claims savings plus monthly fee as a percentage of the comparison group's per beneficiary per month.
NOTES: MHSO is Medicare health support organization. PBPM is per beneficiary per month.
SOURCES: Centers for Medicare & Medicaid Services: Data from Medicare Inpatient, Outpatient, and Physician/Supplier Claims, 2004-2006; MHSO protocol 6.0, terms and conditions.
Figure 2's dark bars show the savings on Medicare payments through each pilot's first 6 months. Savings, as a percentage of comparison group monthly PBPMs, ranged from -3.7 percent (dissavings) to +6.3 percent. MHSO 6 had recovered nearly one-half of its monthly fee plus 5 percent through Medicare payment savings after 6 months. MHSO 3 had covered 31 percent of its required savings. MHSOs 4 and 5 had covered roughly one-quarter of required savings while MHSOs 1 and 2 had saved only 9-16 percent of required savings. Two MHSOs actually experienced Medicare dissavings that will require a turnaround in performance over the next 2½ years.
Discussion
While these findings are based on a short evaluation period and are considered preliminary, they provide insights into what would have to occur over a longer period to meet the original quality and cost savings requirements.
Although the intervention and comparison groups were similar at randomization and start date with respect to beneficiary demographic and clinical characteristics, our analyses revealed some PBPM differences between intervention and comparison groups at the start of the MHS pilot. Beneficiaries who agreed to participate tended to be a healthier and less costly subset of the intervention group. The pilot's design that required MHSOs to recruit beneficiaries may have caused selection of less ill beneficiaries, and it may be difficult to achieve substantial savings by working with a healthier, less costly, participant group. Consequently, CMS gave each MHSO the option of having its intervention PBPM adjusted downwards if its base year intervention PBPM exceeded its comparison PBPM. Moreover, the ultimate adjustment by the financial reconciliator will be different than ours when, according to contract protocols, it uses a more complete claims file and does not weight base year PBPMs by a beneficiary's eligible fraction of days after the start date.
Beneficiaries in general were far more likely to be hospitalized for a condition other than HF or diabetes. It appears that both disease groups are regressing to the mean with fewer subsequent admissions for the condition that qualified beneficiaries for the program. MHSOs may have substantially overestimated the success of their intervention in reducing other hospital admissions—at least relative to a randomly matched comparison group also regressing-to-the-mean. Unless the programs can target and prevent hospitalizations for causes other than HF and diabetes, projected cost savings related to reduced hospitalizations are unlikely.
As a consequence of failing to materially reduce hospitalization rates, management fees paid after 6 months still substantially exceeded any savings produced. All eight MHSOs, in their CMS contracts, predicted that they would achieve budget neutrality after month 12. Without a substantial reduction in each MHSO's monthly fee and/or accelerated savings rates after 6 months, it is questionable whether many MHSOs can meet the pilot's budget neutrality savings expectation within year 1 and the additional 5-percent savings requirement within year 3.
Future analyses will examine a longer intervention period and more critically examine the difficulties that MHSOs have had in reducing acute hospital admissions, partly for reasons unrelated to HF or diabetes. Also, we will explore whether there are subpopulations that benefit most from the interventions. We will examine improvements in additional quality of care measures and reductions in hospitalizations and costs as a function of the length and nature of participation as well as the extent of engagement with individual beneficiaries.
Acknowledgments
We would like to thank Mary Kapp for her comments on our program description and methods as well as comments from three anonymous reviewers.
Footnotes
The authors are with Research Triangle Institute International. The research in this article was supported by the Centers for Medicare & Medicaid Services (CMS) under Contract Number 500-00-0022. The statements expressed in this article are those of the authors and do not necessarily reflect the views or policies of Research Triangle Institute International or CMS.
The HCC score is used in the Medicare Program to adjust managed care payments. A beneficiary with an HCC score of 1.35 is predicted to have Medicare payments next year that are 35 percent greater than estimated payments for the average FFS beneficiary.
Reprint Requests: Jerry Cromwell, Ph.D., Research Triangle Institute International, 1440 Main Street, Suite 310, Waltham, MA 02451. E-mail: jcromwell@rti.org
References
- Anderson G. Chronic Conditions: Making the Case for Ongoing Care. Partnership for Solutions, Johns Hopkins University and the Robert Wood Johnson Foundation; Baltimore, MD.: Dec, 2002. [Google Scholar]
- Bodenheimer T, Wagner EH, Grumbach K. Improving Primary Care for Patients with Chronic Illness: The Chronic Care Model, Part 2. Journal of the American Medical Association. 2002 Oct;288(15):1909–1914. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- U.S. Congressional Budget Office. An Analysis of the Literature on Disease Management Programs. Congressional Budget Office; Washington, DC.: Oct 13, 2004. [Google Scholar]
- Crippen DL. Disease Management in Medicare: Data Analysis and Benefit Design Issues. 2002 Sep 19; Congressional Budget Office Testimony before the Special Committee on Aging, U.S. Senate. Available at: http//www.cbo.gov/doc.cfm?index=3776 (Accessed 2008.)
- Disease Management Association of America. Definition of Disease Management. 2005 Internet address: http://www.dmaa.org/definition.html (Accessed 2008.)
- Foote S. Population-Based Disease Management Under Fee-For-Service Medicare. Web Exclusive. Health Affairs. 2003 Jul 30;:342–356. doi: 10.1377/hlthaff.w3.342. Internet address: http://www.healthaffairs.org (Accessed 2008.) [DOI] [PubMed]
- Goetzel RZ, Hawkins K, Ozminkowski RJ, et al. The Health and Productivity Cost Burden of the “Top 10” Physical and Mental Health Conditions Affecting Six Large U.S. Employers in 1999. Journal of Occupational and Environmental Medicine. 2003;45(1):5–14. doi: 10.1097/00043764-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Goetzel RZ, Ozminkowski RJ, Sederer LI, et al. The Business Case for Quality Mental Health Services: Why Employers Should Care about the Health and Well-Being of Their Employees. Journal of Occupational and Environmental Medicine. 2002;44(4):320–330. doi: 10.1097/00043764-200204000-00012. [DOI] [PubMed] [Google Scholar]
- Goetzel RZ, Ozminkowski RJ, Villagra VG, et al. Return on Investment in Disease Management: A review. Health Care Financing Review. 2005 Summer;26(4):1–19. [PMC free article] [PubMed] [Google Scholar]
- Heaney CA, Goetzel RZ. A Review of Health-Related Outcomes of Multi-Component Worksite Health Promotion Programs. American Journal of Health Promotion. 1997 Mar-Apr;11(4):290–307. doi: 10.4278/0890-1171-11.4.290. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academy Press; Washington, DC.: 2001. [Google Scholar]
- Jencks SF, Huff E, Cuerdon T. Change in the Quality of Care Delivered to Medicare Beneficiaries. Journal of the American Medical Association. 2003;289(3):305–312. doi: 10.1001/jama.289.3.305. [DOI] [PubMed] [Google Scholar]
- Knight K, Badamgarav E, Henning JM, et al. A Systematic Review of Diabetes Disease Management Programs. American Journal of Managed Care. 2005 Apr;11:242–250. [PubMed] [Google Scholar]
- Mattke S, Seid M, Ma S. Evidence for the Effect of Disease Management: Is $1 Billion a Year a Good Investment? American Journal of Managed Care. 2007 Dec;13(12):670–676. [PubMed] [Google Scholar]
- McCall NM, Cromwell J, Burton J, et al. Evaluation of Phase I of Medicare Health Support (Formerly Voluntary Chronic Care Improvement) Pilot Program under Traditional Fee-for-Service Medicare: First Annual Report. RTI International; Jun, 2007. Report to Congress. [Google Scholar]
- McGlynn EA, Asch SM, Adams J, et al. The Quality of Health Care Delivered to Adults in the United States. New England Journal of Medicine. 2003;348(26):2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- Medicare Prescription Drug, Improvement, and Modernization Act. 2003. Public Law 108-173.
- Norris SL, Nichols PJ, Caspersen CJ, et al. The Effectiveness of Disease and Case Management for People with Diabetes: A Systematic Review. American Journal of Preventive Medicine. 2002;22(4S):15–38. doi: 10.1016/s0749-3797(02)00423-3. [DOI] [PubMed] [Google Scholar]
- Rich MW, Beckham V, Wittenberg C, et al. A Multidisciplinary Intervention to Prevent the Re admission of Elderly Beneficiaries with Congestive Heart Failure. The New England Journal of Medicine. 1995 Nov;333(18):1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- Short AC, Mays GP, Mittler J. Center for Studying Health System Change; 2005. Disease Management: A Leap of Faith to Lower-Cost, Higher-Quality Health Care, 2003. Issue Brief Number 69. Internet address: http://www.hschange.com/CONTENT/607/ (Accessed 2008.) [PubMed] [Google Scholar]
- Smith B, Forkner E, Zaslow B, et al. Disease Management Produces Limited Quality-of-Life Improvements in Patients with Congestive Heart Failure: Evidence from a Randomized Trial in Community-Dwelling Beneficiaries. American Journal of Managed Care. 2005 Nov;11(11):701–713. [PubMed] [Google Scholar]
- Thorpe K, Howard DH. The Rise in Spending among Medicare Beneficiaries: The Role of Chronic Disease Prevalence and Changes in Treatment Intensity. Web Exclusive. Health Affairs. 2006 Aug 22;:378–388. doi: 10.1377/hlthaff.25.w378. Internet address: http://www.healthaffairs.org (Accessed 2008.) [DOI] [PubMed]
- Todd W, Nash T, editors. Disease Management, A Systems Approach to Improving Patient Outcomes. Jossey-Bass Publishers; New York: 2001. [Google Scholar]
- Vladeck B. You Can't Get There from Here: Obstacles to Improving Care of the Chronically Ill. Health Affairs. 2001 Nov-Dec;20(6):64–78. doi: 10.1377/hlthaff.20.6.175. [DOI] [PubMed] [Google Scholar]
- Wagner EH, Sandhu N, Newton K, et al. Effect of Improved Glycemic Control on Health Care Costs and Utilization. Journal of the American Medical Association. 2001 Jan;285(2):182–189. doi: 10.1001/jama.285.2.182. [DOI] [PubMed] [Google Scholar]