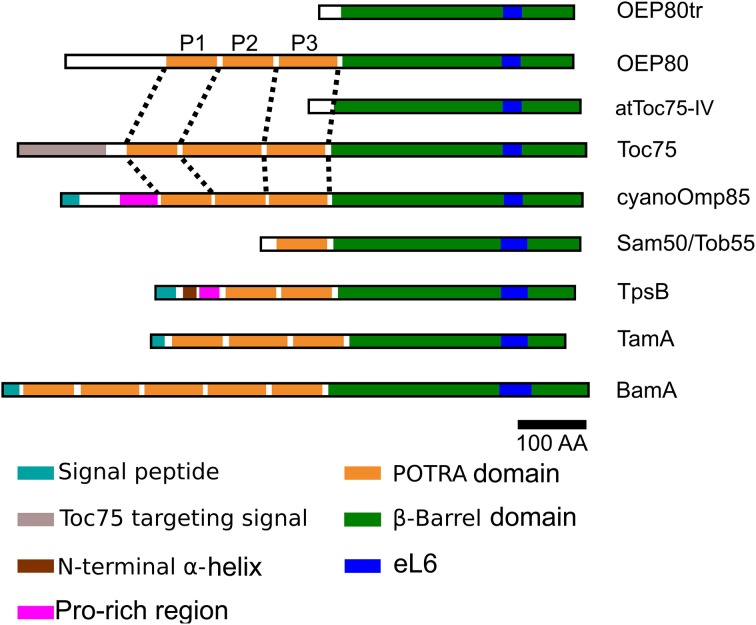
Figure 1.
Molecular architecture of the Omp85 family proteins. Omp85 homologs are comprised of an N-terminal soluble portion containing a variable number of POTRA domains (orange) followed by a C-terminal transmembrane β-barrel (green). The POTRA domains 1, 2, and 3 of the chloroplast and cyanobacteria homologs are indicated as P1, P2, and P3, respectively. Unlike the others, OEP80tr and Toc75-IV do not contain any full POTRA domains although they both contain sequences that align well with the last β-strand of P3 in their relatives. Within the β-barrel that is made of 16 transmembrane β-strands, strands 11 and 12 are separated by the 6th extracellular loop (blue) which contains a sequence highly conserved between all Omp85 homologs. The homologs from bacteria contain a signal peptide (turquoise) that is required for protein export from the cytoplasm to the periplasm. Toc75 contains a unique bipartite targeting signal (gray) at its N terminus and an apparent unique insertion at the beginning of its second POTRA domain. Cyanobacterial Omp85 homologs and TpsB contain a Pro-rich region (pink) N terminus to the first POTRA domain. In addition, TpsB contains an α-helix (brown) N terminus to the Pro-rich region. The domain lengths are approximately to scale.