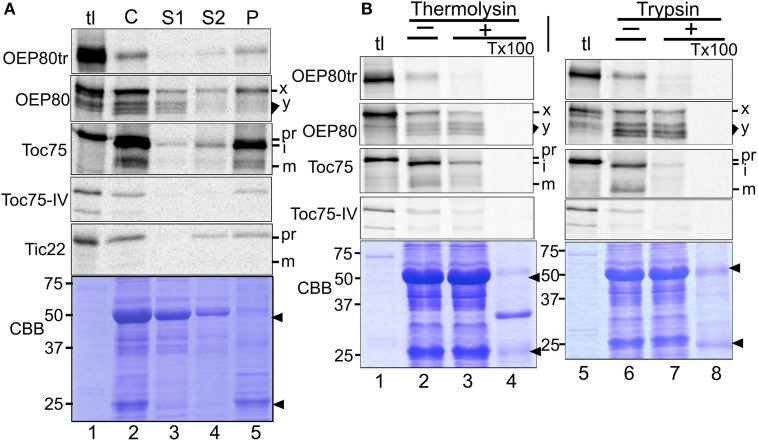
Figure 4.
Import of chloroplast Omp85 homologs in vitro. (A) Chloroplasts isolated from pea seedlings were incubated with radiolabeled proteins indicated at left in the import condition for 10 min in the light. After the reaction, chloroplasts were re-isolated and divided into two samples. The first sample was loaded directly on SDS-PAGE (C). The second sample was lysed hypotonically and separated into supernatant (S1) and pellet fractions by centrifugation. The pellet fraction was further resuspended into 0.1M Na2CO3 and separated into the supernatant (S2) and pellet (P) fractions by another centrifugation. The obtained S1, S2, and P fractions contained soluble, peripheral membrane, and integral membrane proteins, respectively. Radiolabeled proteins recovered in each fraction were separated by SDS-PAGE and visualized by phosphorimager analysis. Each lane was loaded with the sample equal to chloroplasts containing 3 μg chlorophylls used for the import assay, and tl was loaded with 10% of the translation products equivalent to those used for the import assay containing 3 μg chlorophyll-equivalent chloroplasts. For OEP80, the 74-kD OEP80 precursor, and the 66–71 kD import products are indicated with x and y, respectively. For others, pr, i, and m indicate precursor, import intermediate, and mature forms, respectively. The Coomassie Brilliant Blue (CBB)-stained gel for OEP80tr is shown in the bottom. The major soluble protein of 50 kD (LSU = ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit) and the major membrane protein of 25 kD (LHCP = light-harvesting chlorophyll a/b binding protein) are indicated with arrowheads. (B) Chloroplast isolated from pea seedlings were incubated with radiolabeled proteins indicated at left in the import condition for 30 min in the light. After the reaction, chloroplasts were re-isolated and treated for 30 min without (–) or with (+) thermolysin on ice or trypsin at room temperature. Digestion control was done by including detergent (Triton X-100 = TX100) in the reaction. After the reactions were quenched with EDTA (for thermolysin) or trypsin inhibitor (for trypsin), chloroplasts were re-isolated by a 40% Percoll cushion and the radiolabeled proteins in the resultant samples were examined as described in the legend to panel (A). CBB-stained gels for OEP80tr are shown. See legend to panel (A) for descriptions of the labels.