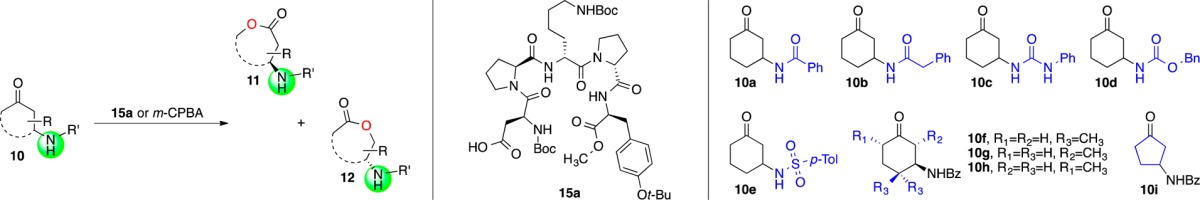
Table 2. Effect of Substituents and Ring Size on Selectivitya.
| reversal
of regioselectivity: regioisomer ratio
(11:12)b |
erb |
|||||||
|---|---|---|---|---|---|---|---|---|
| entry | substrate | conv. (%)c | m-CPBAd | (15a, total) | 15a, matchede | 10 | 11 | 12 |
| 1 | 10a | 41 | 7.9:1 | (1:3.7) | 1:12 | 73:27 | 70:30 | 96:4 |
| 2 | 10b | 45 | 2.5:1 | (1:4.5) | 1:28 | 76:24 | 85:15 | 97:3 |
| 3 | 10c | 64 | 1.4:1 | (1:1.8) | 1:24 | 71:29 | 93:7 | 92:8 |
| 4 | 10d | 20f | 3.0:1 | (1:3.1) | 1:8.2 | NDg | 64:36 | 95:5 |
| 5 | 10e | 20 | 6.1:1h | (1:1.8) | 1:4.4 | 56:44 | 64:36 | 91:9 |
| 6 | 10f | 44 | 5.2:1 | (1:7.3) | 1:23 | 80:20 | 71:29 | 93:7 |
| 7i | 10g | 51 | 1:2.3 | (1:38) | <1:100 | 85:15 | 78:22 | 85:15 |
| 8 | 10h | 52f | >100:1 | (5.2:1) | 2.3:1 | 54:46 | 56:44 | 99:1 |
| 9 | 10i | 44f | 3.3:1 | (1.2:1) | 1:4.3 | 53:47 | 83:17 | 88:12 |
Conditions: 15a (10 mol %), 4-dimethylaminopyridine (DMAP, 10 mol %), N,N′-diisopropylcarbodiimide (DIC, 3.0 equiv), H2O2 (3.8 equiv), CHCl3 (0.1 M), 21 °C, 24 h.
Determined by HPLC.
See Table 1, footnote b.
m-CPBA (1.1 equiv), CHCl3 (0.1 M), 21 °C, 12 h.
Product ratio for the enantiomer that reacts more rapidly with the catalyst.
Conversion approximated from comparison of HPLC peak integrations of the products to the substrate.
ND = not determined.
m-CPBA (2.0 equiv) used.
DIC (1.5 equiv), H2O2 (1.9 equiv) used.
