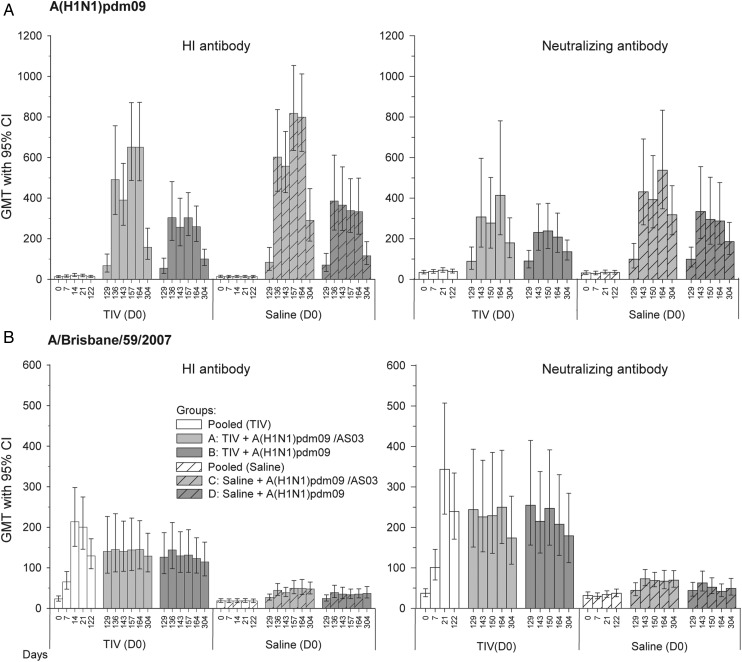
Figure 2.
A(H1N1)pdm09-specific and A/Brisbane/59/2007-specific hemagglutination inhibition (HI) and neutralizing antibody (NAb) responses. A(H1N1)pdm09-specific (panel A) and A/Brisbane/59/2007-specific (panel B) HI and NAb responses shown are of the according-to-protocol cohort for immunogenicity (ATP-I cohort) at D164, except those for day 304 (from ATP-I cohort at day 304). Group A received trivalent seasonal influenza vaccine (TIV) at day 0 and A(H1N1)pdm09 (3.75 µg)/AS03 at day 122 and day 143. Group B received TIV at day 0 and A(H1N1)pdm09 (15 µg) at day 122 and day 143. Group C received saline at day 0 and A(H1N1)pdm09 (3.75 µg)/AS03 at day 122 and day 143. Group D received saline at day 0 and A(H1N1)pdm09 (15 µg) at day 122 and day 143. Abbreviations: CI, confidence interval; GMT, geometric mean titer.