Abstract
Objective
To examine the maturational changes in proteomic polymorphisms resulting from differential expression by multiple intrinsic fungi in the caterpillar body and stroma of natural Cordyceps sinensis (Cs), an integrated micro-ecosystem.
Methods
The surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF MS) biochip technique was used to profile the altered protein compositions in the caterpillar body and stroma of Cs during its maturation. The MS chromatograms were analyzed using density-weighted algorithms to examine the similarities and cluster relationships among the proteomic polymorphisms of the Cs compartments and the mycelial products Hirsutella sinensis (Hs) and Paecilomyces hepiali (Ph). Results: SELDI-TOF MS chromatograms displayed dynamic proteomic polymorphism alterations among samples from the different Cs compartments during maturation. More than 1,900 protein bands were analyzed using density-weighted ZUNIX similarity equations and clustering methods, revealing integral polymorphism similarities of 57.4% between the premature and mature stromata and 42.8% between the premature and mature caterpillar bodies. The across-compartment similarity was low, ranging from 10.0% to 18.4%. Consequently, each Cs compartment (i.e., the stroma and caterpillar body) formed a clustering clade, and the 2 clades formed a Cs cluster. The polymorphic similarities ranged from 0.51% to 1.04% between Hs and the Cs compartments and were 2.8- to 4.8-fold higher (1.92%–4.34%) between Ph and the Cs compartments. The Hs and Ph mycelial samples formed isolated clades outside of the Cs cluster.
Conclusion
Proteomic polymorphisms in the caterpillar body and stroma of Cs change dynamically during maturation. The proteomic polymorphisms in Hs and Ph differ from those in Cs, suggesting the presence of multiple Cs-associated fungi and multiple Ophiocordyceps sinensis genotypes with altered differential protein expression in the Cs compartments during maturation. In conjunction with prior mycological and molecular observations, the findings from this proteomic study support the integrated micro-ecosystem hypothesis for natural Cs.
Introduction
For centuries, Cordyceps sinensis has been used as a precious medicinal product in China and other Asian countries and features a broad spectrum of health benefits, including anti-aging and lifespan-extension effects [1]–[3]. (Note: The Latin name Cordyceps sinensis (Berk.) Sacc. is used for both the teleomorph/holomorph of C. sinensis fungus and the wild product indiscriminately [4],[5]. The fungus was re-named Ophiocordyceps sinensis (Berk.) Sung et al. [6]; however, the Latin name for the wild product has remained unchanged. Because a consensus Latin name for the wild product has not been reached by mycological and TCM botanical taxonomists, we have temporarily used the term O. sinensis to refer to the fungus/fungi and continued to use the name C. sinensis to refer to the wild product.) Mycological and molecular approaches have demonstrated that C. sinensis comprises more than 90 intrinsic fungi from more than 37 genera and at least 6 O. sinensis genotypes [7]–[25]. Although an anamorph-teleomorph connection between Hirsutella sinensis and O. sinensis has been proposed based on the aggregation of indirect evidence [4]–[5],[24], integrated analyses have demonstrated large dissimilarities between the random amplified polymorphic DNA (RAPD) polymorphisms of H. sinensis and of the C. sinensis ascocarp and no studies to date have truly satisfied Koch's postulates by describing the successful artificial induction of C. sinensis sexual fruiting bodies and ascospores [9],[17]–[18],[25]–[29]. However, there has been no direct evidence to either approve or reject the Paecilomyces hepiali hypothesis for the O. sinensis anamorph [30]. P. hepiali, H. sinensis and several mutant genotypes of O. sinensis have been found to naturally coexist in the ascocarps and ascospores of natural C. sinensis, and the fungal complex showed a 39-fold enhancement of its infection potency over that of pure H. sinensis [31]–[32]. Other researchers have thus hypothesized that C. sinensis is an integrated micro-ecosystem with differential expressions by multiple intrinsic fungi in its compartments and have identified a culture-dependent microbial community or mycobiota in natural C. sinensis along with evidence of possible symbiotic interactions among the component fungi [13]–[23],[27]–[32]. We have previously reported dynamic changes in the differential fungal expression of at least 6 O. sinensis genotypes during C. sinensis maturation [19]–[23],[29]. However, no previous studies have compared the proteomes of C. sinensis and H. sinensis (the proposed anamorphic fungus of O. sinensis) or reported global changes in the macrocosmic proteomic polymorphisms in C. sinensis compartments during maturation. In contrast to the microcosmic studies that have focused specifically on individual protein species, we used the surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF MS) protein chip technique in this study to macrocosmically profile the changes in proteomic polymorphisms in the C. sinensis caterpillar body and stroma during maturation [33]–[34]. We also examined the similarities and cluster relationships between the proteomic polymorphisms of C. sinensis and those of the mycelial fermentation products H. sinensis Bailing and P. hepiali Cs-4.
Materials and Methods
Collection of C. sinensis
Fresh C. sinensis specimens were purchased in a local market (Latitude 30°04′N, Longitude 101°95′E) in the Kangding County of Sichuan Province, China. Governmental permission was not required for C. sinensis purchases in local markets, and the collections of C. sinensis specimen sales by local farmers fall under the governmental regulations for traditional Chinese herbal products. Premature C. sinensis features a plump caterpillar body (sclerotium) and a short stroma ranging from 1.0 to 2.0 cm in length (Figure 1). Mature C. sinensis features a less plump caterpillar body and a long stroma with a length of>5.0 cm and an expanded portion densely covered with ascocarps close to the stroma tip. All fresh C. sinensis specimens were washed thoroughly on site in running water with gentle brushing, soaked in 0.1% mercuric chloride for 10 min for surface sterilization and washed again 3 times with sterile water. The specimens were immediately frozen in liquid nitrogen for transportation and storage prior to further processing in the lab in Beijing [13].
Figure 1. Freshly collected natural C. sinensis at 2 maturation stages with stromata of various heights.

Sample preparations for proteomic profiling
Ten C. sinensis specimens at each maturation stage were used in this study. The caterpillar bodies and stromata from the premature and mature C. sinensis specimens and the mycelial fermentation products H. sinensis Bailing (Bailing capsule, Lot #040811, #050403 and #051103, Zhejiang American-Sina Pharmaceutical Company, Hangzhou, Zhejiang, China) and P. hepiali Cs-4 (Jinshuibao capsule, Lot #JX12931, #20040608 and #20051020, Jiangxi TCM Pharmaceutical Company, Nanchang, Jiangxi, China) were individually ground into powder in liquid nitrogen. To evaluate the proteomic polymorphisms of the samples as group-averages at each maturation stage to minimize the influence of individual variations due to sampling and the lack of a more accurate method to measure the sample's maturation status, the powders (0.5 g each) of the C. sinensis compartment samples were pooled according to their compartment origins and maturational stages to form the following testing samples: premature stroma, premature caterpillar body, mature stroma and mature caterpillar body. Based on the pre-test results with insignificant variations in overall proteomic similarity, 0.95 for the 3 H. sinensis Bailing samples and 0.96 for the 3 P. hepiali Cs-4 samples, the powders of H. sinensis Bailing (Lot #051103) and P. hepiali Cs-4 (Lot #20051020) were selected for the formal study. The powder samples were dissolved in 600 µl of tris-glycine buffer (pH 8.3) and centrifuged at 14,000 rpm for 5 min at 4°C. The sample supernatants were used for proteomic profiling.
SELDI-TOF mass spectrometry
The supernatants prepared above were diluted in PBS to a concentration of 200–300 nM before application to a normal-phase biochip and analysis on a PBS-II protein chip reader (SELDI-TOF MS; BioSpace Ciphergen Biosystems, Fremont, CA, USA) [33]–[34]. The SELDI-TOF MS experiments were performed at the Universities' Confederated Institute for Proteomics at the School of Life Sciences, Beijing Normal University, Beijing, China. In brief, different proteins captured on the surface of protein chips were collected through SELDI-TOP mass spectrometry using a laser power of 215 (sensitivity 9; molecule size range: 0–60,000 Da). Following mass calibration, total ion current normalization and baseline subtraction, the molecular size ranges of proteins were manually selected for analyses, and the intensities (peak heights) were extracted using ProteinChip software (Ciphergen proteinchip 3.0.2).
Across-chromatogram normalization of densities of protein species
The SELDI-TOF MS chromatograms were scanned with Quantity One software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). To conduct integrated proteomic profiling on the basis of chromatographic tracing at the molecular weight segments, the band trace quantities (OD*mm) of all protein bands in all chromatograms were normalized using the maximal chromatographic tracing scales for each molecular weight segmented tracing panel as the reference factor (Figure 2). The relative intensity/density was defined as the scanned band trace quantity (OD*mm) multiplied by the difference between the maximum scale “n” on the vertical axis of each chromatographic tracing panel and the baseline scale if the trace baseline was not exactly zero.
Figure 2. A representative SELDI-TOF MS chromatogram tracing.
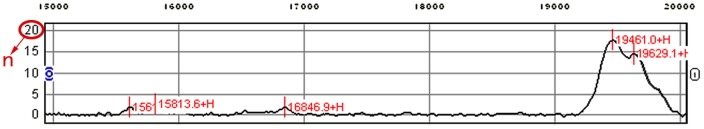
The tracing scale maximum, n, on the vertical axis of each chromatographic panel was used as the across-chromatogram normalization reference.
Similarity computation for proteomic polymorphisms
ZUNIX equations (http://www.ebioland.com/ZUNIX.htm; Beijing Bioland Technology, 2013) were used for similarity computations with band intensities/density weighting [28]–[29] while considering (i) mismatched protein bands and (ii) matched protein bands with dissimilar intensities/densities. The following ZUNIX equation (1) was used to compare the polymorphisms of 2 mass spectrometry chromatograms: dik≥0, i = 1,2, k = 1,2, …, m. We defined the measure of similarity as follows:
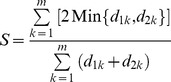 |
(1) |
where the similarity of the 2 densities d 1k and d 2k is defined as the common portion of their values.
The second ZUNIX equation (2) is suitable for comparing the proteomic polymorphisms in more mass spectrometry chromatograms, where dik≥0, i = 1,2,…,n, k = 1,2,…,m and the description is as follows:
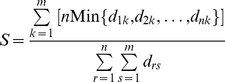 |
(2) |
Density-weighted cluster analysis for the polymorphic protein fingerprints
For the mismatched protein species, a missing band at the given molecular weight location in a MS chromatogram was assigned a score of 0. The digital density data of all matched and unmatched protein bands in the compared chromatograms were ranked and arbitrarily assigned scores of 1–9 according to the ranks of their densities in 2 or more compared chromatograms [28]–[29]. The digital data scores were entered into PAUP 4.0B (Swofford, 2002; Sinauer Asso. Inc, Sunderland, MA, USA) to construct cluster trees (semi-quantitative density-weighted neighbor-joining distance method; bootstrap = 1000). In addition to the semi-quantitative algorithm provided by PAUP 4.0B, a fully quantitative cluster analysis was also performed with a parametric hierarchical clustering analysis (density-weighted furthest neighbor Pearson correlation average linkage distance method) in SPSS 10.1 (SPSS Inc., Chicago, IL, USA; Note: no bootstrap strategy was provided in the software package).
Results
Comparison of the protein fingerprint chromatograms of premature and mature C. sinensis
Figure 3 displays the SELDI-TOF MS chromatograms for the C. sinensis protein species at the 2 maturation stages in a molecular weight range of 0 to>60,000 Daltons. Using the density-weighted ZUNIX equation (1), a percentage similarity of 57.9% was observed between the protein fingerprint polymorphisms of pooled premature and mature C. sinensis samples, thus indicating altered protein expression during C. sinensis maturation.
Figure 3. SELDI-TOF MS protein chromatograms to examine the protein fingerprints (molecular weight: 0 to>60,000 Daltons) and proteomic polymorphisms of premature and mature C. sinensis.
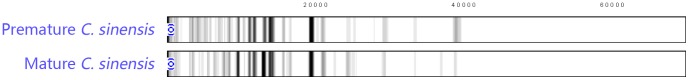
Comparison of the polymorphic protein chromatograms of the caterpillar bodies and stromata of premature and mature C. sinensis
Figure 4 displays the SELDI-TOF MS chromatograms for the C. sinensis caterpillar body and stroma protein moieties at the 2 maturational stages in a molecular weight range of 0 to>60,000 Daltons. Using ZUNIX equation (2), an overall percentage similarity of 3.1% was observed between the proteomic polymorphisms for all C. sinensis caterpillar body and stroma samples at both maturation stages. Using ZUNIX equation (1) for pairwise comparisons, the calculated similarities from Figure 4 were 57.4% and 42.8% between the proteomic polymorphisms of the 2 maturation stages in the stromata or caterpillar bodies, respectively, but were much lower (10.0%–18.4%) for the across-compartment pair comparisons (Table 1). These similarities indicate major differences in the proteomic profile within the C. sinensis caterpillar body and stroma resulting from large differences in the compositions of the multiple intrinsic fungi from more than 37 genera and at least 6 mutant O. sinensis genotypes together with the transcription and translation of their fungal genes. In contrast to the large between-compartment differences in the proteomic profile, the within-compartment differences were moderate across the C. sinensis maturation stages.
Figure 4. SELDI-TOF MS protein chromatograms to examine the protein fingerprints (molecular weight: 0 to>60,000 Daltons) and proteomic polymorphisms of the caterpillar bodies and stromata of premature and mature C. sinensis.
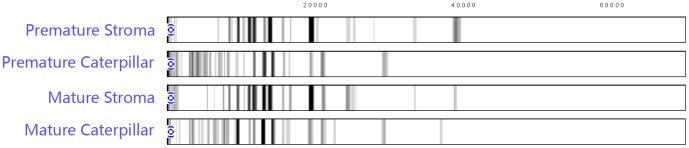
Table 1. Percentage similarities in the total protein profiles of the caterpillar bodies and stromata of the premature and mature C. sinensis, computed with the density-weighted ZUNIX equation (1).
| Stroma | Caterpillar Body | |||
| Premature | Mature | Premature | Mature | |
| Premature stroma | — | — | — | — |
| Mature stroma | 57.4% | — | — | — |
| Premature caterpillar body | 10.0% | 13.2% | — | — |
| Mature caterpillar body | 18.4% | 17.8% | 42.8% | — |
Comparison of the C. sinensis, H. sinensis and P. hepiali sample protein fingerprint chromatograms in multiple molecular weight segments
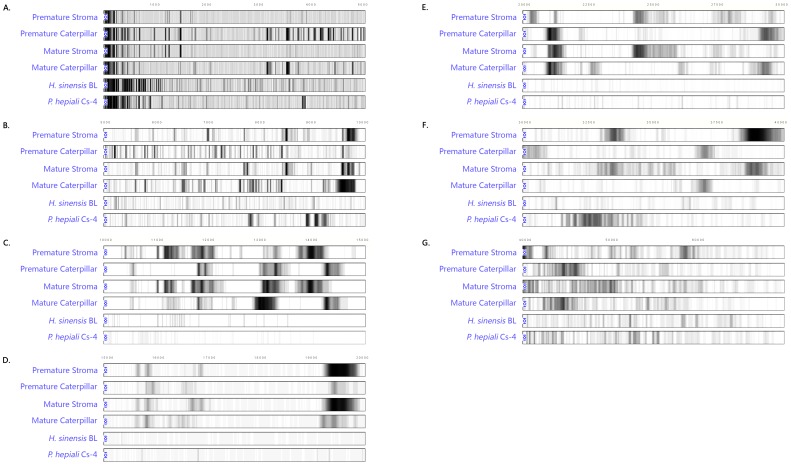
The above-described results for the C. sinensis proteins are displayed integrally from molecular weights of 0 to>60,000 Daltons, as shown above in Figures 3 and 4. To increase the chromatographic resolution, Figure 5 displays 7 panels of the segmented SELDI-TOF MS chromatograms of all protein species in the C. sinensis caterpillar bodies and stromata at the 2 maturation stages as well as of the commercial mycelial fermentation products H. sinensis Bailing and P. hepiali Cs-4. Figure 5-A presents protein species in the molecular weight range from 0 to 5,000 Daltons, Figure 5-B ranges from 5,000 to 10,000 Daltons, Figure 5-C ranges from 10,000 to 15,000 Daltons, Figure 5-D ranges from 15,000 to 20,000 Daltons, Figure 5-E ranges from 20,000 to 30,000 Daltons, Figure 5-F ranges from 30,000 to 40,000 Daltons and Figure 5-G ranges from 40,000 to>60,000 Daltons.
Figure 5. SELDI-TOF MS protein chromatograms to examine protein polymorphisms in the stroma and the caterpillar body specimens of premature and mature C. sinensis and the mycelial fermentation products H. sinensis Bailing and P. hepiali Cs-4.
Total proteins were extracted from the caterpillar bodies or stromata of natural C. sinensis at 2 maturation stages and from the mycelial products H. sinensis Bailing (BL) or P. hepiali Cs-4. Panels A, B, C, D, E, F and G present proteins with molecular weights ranging from 0–5,000, 5,000–10,000, 10,000–15,000, 15,000–20,000, 20,000–30,000, 30,000–40,000 and 40,000 to>60,000 Daltons, respectively.
The segmented mass spectrometry chromatograms shown in Figure 5 indicate large polymorphic differences between the protein profiles of the C. sinensis compartments at each of the 2 maturation stages, and the complex protein expression patterns resulting from multiple intrinsic fungi across the C. sinensis compartments underwent differential maturational fungal expression changes. The mass spectrometry chromatograms also indicate large overall differences between the proteomic polymorphisms of C. sinensis and those of the fermentation products H. sinensis Bailing and P. hepiali Cs-4.
Polymorphic similarities in the protein fingerprints of the C. sinensis compartments at 2 maturational stages and the mycelial fermentation products H. sinensis Bailing and P. hepiali Cs-4
The densities of all protein species in all 7 mass spectrometry chromatogram panels shown in Figure 5 were normalized using the mass spectrometry tracing scales described in the Methods section and illustrated in Figure 2. The normalized densities were subjected to polymorphic similarity calculations with ZUNIX similarity equation (1) [28]–[29], to examine the similarities between the protein profiles of the C. sinensis compartment and those of the mycelial products H. sinensis Bailing and P. hepiali Cs-4. As shown in Table 2, the proteomic polymorphism similarities were low (0.51%–1.04%) between the H. sinensis Bailing and C. sinensis protein profiles and were 2.8- to 4.8-fold higher (1.92%–4.34%) between the P. hepiali Cs-4 and C. sinensis compartments than between the H. sinensis Bailing and C. sinensis compartments.
Table 2. Percentage similarities in proteomic polymorphisms between the C. sinensis compartment samples at 2 maturational stages and H. sinensis Bailing (BL) and P. hepiali Cs-4.
| Stroma | Caterpillar body | |||||
| Premature | Mature | Premature | Mature | H. sinensis BL | ||
| Percentage similarity (%) | H. sinensis BL | 0.69% | 0.51% | 1.04% | 0.87% | — |
| P. hepiali Cs-4 | 1.92% | 2.33% | 4.34% | 4.18% | 6.52% | |
| Similarity Ratio (fold) | (P. hepiali Cs-4 vs. H. sinensis BL) | 2.8-fold | 4.5-fold | 4.2-fold | 4.8-fold | |
Density distributions of all protein bands and determination of the scoring cutoff value for the semi-quantitative analysis
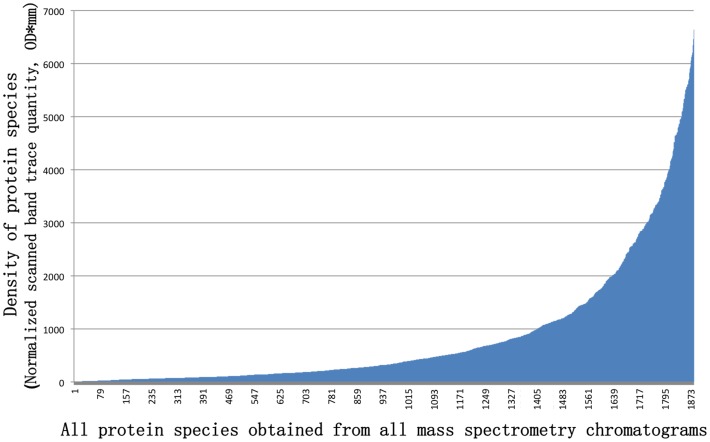
After normalization, the scanned band trace quantities (OD*mm) of approximately 1,900 protein bands were sorted for arbitrary scoring in preparation for the cluster construction using the semi-quantitative density-weighted algorithm (Figure 6).
Figure 6. Distribution of the normalized scanned band trace quantities (OD*mm) of approximately 1,900 protein species from all segmented SELDI-TOF MS protein chromatograms in Figure 5 .
Density-weighted cluster analysis of the protein fingerprints of C. sinensis and the mycelial products H. sinensis Bailing and P. hepiali Cs-4
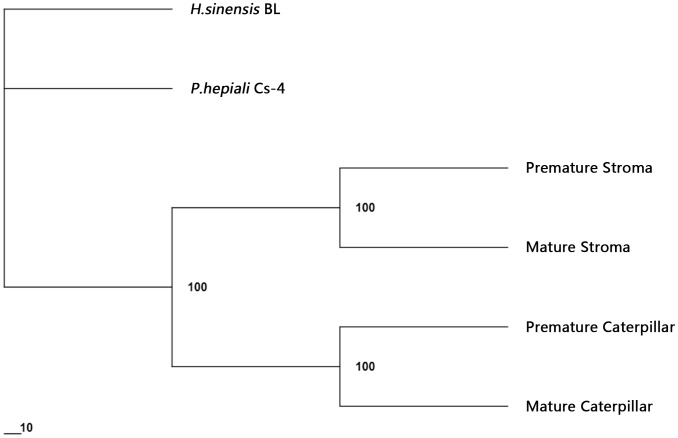
The highest density value in Figure 6 was divided by 9 to obtain the critical cut-off values for a semi-quantitative density grouping. Each density was assigned a score from 1 to 9 according to the above-mentioned cutoff values, and all arbitrarily assigned scores were used for the cluster construction according to the density-weighted algorithm provided by PAUP 4.0B software [28]–[29]. Figure 7 displays a cluster tree that was constructed with the density-weighted neighbor-joining algorithm (bootstrap = 1000). Similar to the percentage similarity results shown in Table 1, the caterpillar body samples formed 1 clade and the stroma samples formed another clade; these 2 clades then formed a C. sinensis cluster. The mycelial fermentation product H. sinensis Bailing formed an isolated clade that was separated from the C. sinensis cluster by the clade formed by P. hepiali Cs-4.
Figure 7. Integral cluster tree of all the proteomic chromatograms constructed with the semi-quantitative density-weighted algorithm.
“H.sinensis BL” refers to the H. sinensis Bailing mycelial product, “P.hepiali Cs-4” refers to the P. hepiali Cs-4 mycelial product, and “Caterpillar” refers to the caterpillar body. Each protein species from all proteomic chromatograms in Figure 5 was assigned a score of 1–9 based on its density rank among the densities of all compared protein species; the missing protein band at the same molecular weight was assigned a score of 0. All protein species from the chromatograms in Figure 5 were entered into the cluster construction using the neighbor-joining distance method (bootstrap = 1000).
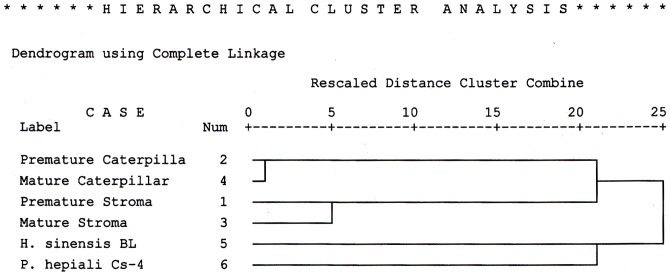
Although the PAUP 4.0B software offered the advantage of constructing cluster trees according to the bootstrap value (bootstrap = 1000), the program used only semi-quantitative algorithms. A fully quantitative, density-weighted algorithm included in the SPSS 10.1 software package was also employed to construct a cluster tree [28]. As shown in Figure 8, the C. sinensis sample clade formation pattern for C. sinensis samples generated using the fully quantitative algorithm was similar to that generated via semi-quantitation, as shown in Figure 7. The caterpillar body and stroma clades joined to form a C. sinensis cluster with a greater rescaled distance in the cluster tree that was indicative of large differences in polymorphic protein expression between the C. sinensis caterpillar bodies and stromata and reflective of the low similarity observed between the C. sinensis compartments in Table 1. The mycelial fermentation products H. sinensis Bailing and P. hepiali Cs-4 formed a clade with a greater rescaled distance and were thus situated outside of the C. sinensis cluster.
Figure 8. Integral cluster tree of all proteomic chromatograms constructed with the fully quantitative density-weighted algorithm.
“H. sinensis BL” refers to the H. sinensis Bailing mycelial product; “Caterpillar” or “Caterpillar” refers to the caterpillar body. All protein species from all chromatograms in Figure 5 were entered into the cluster construction, using the furthest neighbor (Pearson correlation average linkage) method of hierarchical cluster analysis.
Discussion
C. sinensis is one of the most valued Chinese medicinal products. This organism grows only in areas of high elevation on the Qinghai-Tibetan Plateau and features a complex life cycle. Studies have reported that C. sinensis comprises more than 90 intrinsic fungal species from more than 37 genera and at least 6 genotypes of O. sinensis fungi [7]–[23]. Of these, the most abundant culturable fungi are Pseudogymnoascus roseus in the sclerotia and cortices and Penicillium chrysogenum in the stromata, as reported by Zhang et al. [17]. Previously, we reported that C. sinensis maturation was associated with dynamic changes in the intrinsic fungal species and mutant O. sinensis genotypes along with significant changes in the RAPD molecular marker polymorphisms and component chemicals [13],[19]–[23],[29]. The fungal background of C. sinensis becomes even more complex when non-culturable fungal species are considered [18]–[23]. These findings reflect the altered fungal expression of multiple intrinsic fungi and support the hypothesis that C. sinensis is an integrated micro-ecosystem of multiple intrinsic fungi, as proposed by Liang et al. [27]. Density-weighted algorithms for similarity computations and cluster constructions were used in this study to analyze the mass spectrometry chromatograms of polymorphic proteomes, the downstream transcription/translation products of multiple fungal genomes. We observed different proteomic profiles with similarities of 10.0% between the premature caterpillar bodies and stromata and 17.8% between the mature caterpillar bodies and stromata of C. sinensis (cf. Figure 4; Table 1), consistent with the mycological and molecular observations of diverse fungal populations in the two C. sinensis compartments [17]–[18]. However, considerably great proteomic polymorphism similarities of 42.8% and 57.4% were observed within the C. sinensis caterpillar body and stroma, respectively, at the 2 C. sinensis maturation stages (cf. Figure 4; Table 1). The differences in the across and within-compartment similarities between the proteomic profiles might possibly be derived from 2 major factors: (1) differential protein expression of the multiple fungal genomes (multiple mutant O. sinensis genotypes and the multiple intrinsic mesophilic and psychrophilic fungi), of which part or all undergo maturational alterations, and (2) protein species from the dead bodies of the C. sinensis ghost moth larvae, which are not merely a group of nutrients for fungal growth but also as a part of the species complex, along with all of the previously reported small chemical components [13],[38]–[47], contribute to the overall pharmacology of the natural medicinal product and partly explain the various therapeutic potencies of premature and mature C. sinensis that have been identified by traditional Chinese medicine quality grading system.
Density-weighted algorithms for similarity computations and cluster constructions were used to compare RAPD molecular marker polymorphisms in previous studies of C. sinensis [28]–[29]. Although density-unweighted arithmetic methods have been widely used in literature, these methods are only suitable for the analyses of “all-or-none” data. The density-weighted arithmetic methods used in this proteomic study are more mathematically general and sufficiently sensitive to capture all of the detailed information regarding dynamic changes in proteins expressed by the various intrinsic fungi during C. sinensis maturation [28]–[29]. These algorithms provide scientists with accurate analytical means with which to trace changes in the proteomic and molecular marker polymorphisms in natural C. sinensis.
In this first study testing the proteomic polymorphisms of different compartments of wild C. sinensis during maturation, the overall proteomic polymorphisms were compared in the pooled samples of 10 C. sinensis specimens of each maturation stage. The height of the C. sinensis stroma (cf. Figure 1) has been taken as the standard for the potency-quality grading of natural C. sinensis on the market. Such a common practice for potency grading can be explained by a C. sinensis mycology expert that the premature C. sinensis with a short stroma grows asexually, whereas the long-stroma C. sinensis with the formation of the ascocarp portion (the expanded portion close to the tip of the stroma) primarily grows sexually (personal communication with Prof. YL Guo). According to the comments of Prof. Guo regarding the asexual-sexual growth of C. sinensis, our previous molecular systematic studies demonstrated that the maturation of wild C. sinensis is a continuous biological course along with the weather during spring. However, there is no existing accurate method thus far to measure sample's maturation status. We also found previously large differences in the fungal activity of H. sinensis, biomasses of the fungus-specific DNA species, and small organic chemicals in wild C. sinensis during maturation, indicating large differences in fungal expression during C. sinensis maturation [13],[19]–[23],[28]–[29]. Therefore, we designed this study with 2 special sample arrangements of the test materials to minimize variations in individual specimens: (1) the selection of C. sinensis specimens with the clear morphological characters shown in Figure 1, i.e., very premature C. sinensis with a short possible stroma (1-2 cm) and mature C. sinensis with a long stroma (>5 cm) and with definite formation of the ascocarp portion; and (2) assessment of the pooled samples (10 specimens at each maturation stage; stromata and caterpillar bodies separated from the same specimens). In addition to the examination of maturational and compartmental group differences in proteomic polymorphism within a C. sinensis population, it is possible that there are individual differences in some degree in proteomic polymorphism within a C. sinensis population and within maturation groups. These individual differences are likely due to differences such as in the instar and nutrition status of the larvae of ghost moths within the family Hepialidae at the time of fungal infection, the growth location and environment (e.g., elevation and temperature, strength of plateau wind and sunshine in the growth area, amount of snow in winter and rain in spring, soil fertility, surrounding vegetation), the total weight and length of the C. sinensis specimens, and the weight ratio and height ratio of the caterpillar body and stroma of individual C. sinensis specimens. This study design of pooling samples, however, is limited regarding the exploration of such individual variations at each estimated maturation stage. However, there may also be population differences among the C. sinensis specimens collected from different production areas, likely due to the different species of larvae of ghost moths within the family Hepialidae, differences in latitude, possibly different local soil fungal flora or mycobiota and other environment factors. All these considerations should encourage future studies to further explore variations in the molecular and proteomic polymorphisms and chemical profiles among the individual C. sinensis specimens collected within a production area or among the C. sinensis populations from various production areas. Perhaps prior to the future comparison of individual specimens, an accurate method for determining C. sinensis maturation stages may need to be established with the combined use of morphological characters and molecular markers to distinguish proteomic variations due to slightly different maturation stages of C. sinensis specimens or due to true differences in protein expression in individual specimens at the same maturation stage. To this end, fungal biomass ratios, for example, GC-biases vs. AT-biases of O. sinensis, may serve as a molecular marker to assist the morphological characterization when determining the C. sinensis maturation status [15]–[16],[19]–[23].
H. sinensis has been proposed as an anamorph of O. sinensis; natural C. sinensis is considered a single fungus product [4]–[5]. These hypotheses were proposed based on the aggregation of indirect evidence, such as morphological findings for the isolates from natural C. sinensis, ITS sequencing and results from microcycle conidiation of ascospores under particular culture conditions [4]–[5],[24]. No scientific studies to date have truly satisfied Koch's postulates, which have demonstrated the successful artificial induction of the C. sinensis sexual fruiting body and ascospores [5],[9],[24]–[27],[35]. Shen et al. [36] reported extremely slow growth (approximately 2 cm after 7 months) of artificial Cephalosporium dongchongxiacae (≡ H. sinensis; [4],[36]) fruiting bodies and observed regular, fine and deep twills on the surfaces of long, conically shaped fruiting bodies. The overall appearance of the artificial fruiting bodies, unfortunately, was distinct from that of natural C. sinensis, which has a long, round and cylindrical stroma with vertical fine wrinkles, as described in the Chinese Pharmacopoeia. Shen et al. [36] also reported the production of ascospores from one of the artificial C. dongchongxiacae fruiting bodies that featured no morphological formation of a C. sinensis-like ascocarp, the sexual organ of C. sinensis, thus indicating an overall teleomorphic morphology distinct from that of natural C. sinensis. Shen et al. [36] characterized in their paper the unique teleomorphic features of C. dongchongxiacae, and the results actually negated the anamorph-teleomorph connection between C. dongchongxiacae (≡ H. sinensis) and O. sinensis in accordance with Koch's postulates. In addition to the dramatic dissimilarities in the RAPD molecular marker polymorphisms between the C. sinensis ascocarp and H. sinensis, the drastically different proteomic polymorphisms in C. sinensis and H. sinensis might not support the “single-fungus” hypothesis of C. sinensis or the hypothesis of an anamorph-teleomorph connection between H. sinensis and O. sinensis [13],[25],[28]–[29],[37] (cf. Figure 5 and Table 2). Based on these microcosmic and macrocosmic studies, Liang et al. [27] hypothesized that C. sinensis is an integrated micro-ecosystem with varying compositions of multiple intrinsic fungi. In fact, the coexistence of these multiple fungi has been demonstrated in the culture-dependent and independent microbial communities or mycobiota present in natural C. sinensis, along with evidence of symbiotic interactions among the component fungi; these likely represent the key biological actions essential to the natural or artificial production of sexual fruiting bodies and ascospores [17]–[18],[31]–[32].
Studies of C. sinensis have detected several groups of chemical components, including carbohydrates; galactomannan; nucleosides; proteins, polypeptides, oligopeptides, polyamines, and diketopiperazines (cyclo-dipeptides); non-hormone sterols; fatty acids and other organic acids; and vitamins and inorganic elements [1],[38]–[40]. Other compounds such as verticiol, acid deoxyribonuclease, myriocin, 3-deoxyadenosine (coydycepin) and cordysinins A–E have also been detected [41]–[45]. Chemical constituent fingerprinting techniques have been used together with similarity comparisons and cluster constructions in C. sinensis studies to demonstrate the high similarity of C. sinensis samples collected from different production areas [13],[38],[40]–[47]. A cluster analysis of these small organic chemicals via capillary electrophoresis technology demonstrated that several mycelial fermentation products were situated in different clades outside of the cluster containing the natural C. sinensis samples collected from different production areas on Qinghai-Tibetan Plateau [40]. However, when analyzing the small chemicals of several natural C. sinensis samples collected from Tibet or Qinghai Provinces and of fermentation products (P. hepiali Cs-4 and H. sinensis Bailing) through HPLC fingerprinting, the clades of natural C. sinensis were much closer in rescaled distance to the clade containing the P. hepiali Cs-4 products than to the clade containing the H. sinensis Bailing products [38]. In the proteomic fingerprint analysis conducted in the current study, in which the bootstrap strategy (bootstrap = 1,000) was used in the density-weighting algorithm, the fermented products P. hepiali Cs-4 and H. sinensis Bailing were situated in an isolated clade outside of the C. sinensis cluster with the possibility that P. hepiali Cs-4 was closer to the C. sinensis cluster than was H. sinensis Bailing, as shown in Figure 7. This possibility was supported by the 2.8- to 4.8-fold higher similarities of the proteomic polymorphisms between the P. hepiali Cs-4 and C. sinensis compartments relative to those between the H. sinensis Bailing and C. sinensis compartments (cf. Table 2). The cluster relationship demonstrated by the semi-quantitative neighbor-joining algorithm was validated using the fully quantitative approach of the furthest neighbor algorithm without the bootstrap strategy provided by the SPSS software (cf. Figure 8).
Local herbal farmers in the C. sinensis production areas of the Qinghai-Tibetan Plateau have long recognized the temperature dependency of the C. sinensis maturational features and believe that “eating ‘worms’ in the winter and ‘grass’ in the summer” provides tonic herbal properties. Changes in the therapeutic properties of C. sinensis during its maturation have also been recognized by the field of traditional Chinese medicine, in which natural C. sinensis is graded accordingly. We have reported maturational changes in the composition of multiple intrinsic fungal species of C. sinensis and at least 6 genotypes of O. sinensis, along with environmental changes (temperature, sunlight intensity, snow/rain, moisture and plateau wind) on the Qinghai-Tibetan Plateau [13],[19]–[23]. The altered fungal background of natural C. sinensis at various maturation stages causes large variations in (i) the RAPD molecular marker polymorphisms, (ii) the fingerprints of small organic compounds, and (iii) proteomic polymorphisms in the caterpillar bodies and stromata, as demonstrated in this and previous studies [13],[19]–[23],[28]–[29]. The integration of the component compounds that are differentially expressed in different compartments of C. sinensis and differentially altered during C. sinensis maturation constitutes the dynamic pharmacological base that is responsible for the varying potencies of the health benefits and therapeutic activities associated with C. sinensis.
In conclusion, SELDI-TOF MS proteomic profiling was used to macrocosmically detect the dynamic polymorphic alterations among differentially expressed proteins in the different C. sinensis compartments during maturation. The apparent proteomic polymorphism dissimilarity between H. sinensis and C. sinensis suggests different fungal backgrounds of these organisms and thus might not support the “single-fungus” hypothesis of C. sinensis or the hypothesis of an anamorph-teleomorph connection between H. sinensis and natural C. sinensis. However, the findings from this proteomic study, in corroboration with prior mycological and molecular observations, support the integrated micro-ecosystem hypothesis for natural C. sinensis.
Acknowledgments
The authors are grateful to Prof. P.Y. Xu, Mr. W. Chen, Ms. M. Yang and Mr. Y.C. Zhou for their assistance during the collection of the wild C. sinensis samples.
Funding Statement
This study was supported by the Pharmanex Cordyceps sinensis Research Fund. The funder provided support in the form of full or partial salaries for the authors [YZD, LJZ, ZMW, LG, YSY, NZT, JSZ], the purchase of research materials and the payment of SELDI-TOF MS service fees. The funder did not have any additional role in the study design, data collection and analyses, decision to publish or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1. Zhu J-S, Halpern GM, Jones K (1998a) The scientific rediscovery of a precious ancient Chinese herbal regimen: Cordyceps sinensis: Part I. J Altern Complem Med. 4(3): 289–303. [DOI] [PubMed] [Google Scholar]
- 2. Zhu J-S, Halpern GM, Jones K (1998b) The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis: Part II. J Altern Complem Med 4(4): 429–457. [DOI] [PubMed] [Google Scholar]
- 3. Tan NZ, Berger JL, Zhang Y, Prolla TA, Weindruch R, et al. (2011) The lifespan-prolonging effect of Cordyceps sinensis Cs-4 in normal mice and its molecular mechanisms. FASEB J 25(1): 599.1. [Google Scholar]
- 4. Wei XL, Yin XC, Guo YL, Shen NY, Wei JC (2006) Analyses of molecular systematics on Cordyceps sinensis and its related taxa. Mycosystema 25(2): 192–202. [Google Scholar]
- 5. Guo YL, Xiao PG, Wei JC (2010) On the biology and sustainable utilization of the Chinese Medicine treasure Ophiocordyceps sinensis (Berk.) G. H. Sung et al. Modern Chin Med 12(11): 3–8. [Google Scholar]
- 6. Sung GH, Hywel-Jones NL, Sung JM, Luangsa-Ard JJ, Shrestha B, et al. (2007) Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol 57(1): 5–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao J, Wang N, Chen YQ, Li TH, Qu LH (1999) Molecular identification for the asexual stage of Cordyceps sinensis. Acta Sci Natural Univ Sunyatseni 38(1): , 122–123.
- 8. Chen YQ, Wang N, Qu LH, Li TH, Zhang WM (2001) Determination of the anamorph of Cordyceps sinensis inferred from the analysis of the ribosomal DNA internal transcribed spacers and 5.8S rDNA. Biochem Syst Ecol 29: 597–607. [DOI] [PubMed] [Google Scholar]
- 9. Jiang Y, Yao YJ (2003) A review for the debating studies on the anamorph of Cordyceps sinensis . Mycosistema 22(1): 161–176. [Google Scholar]
- 10. Chen YQ, Hu B, Xu F, Zhang WM, Zhou H, et al. (2004) Genetic variation of Cordyceps sinensis, a fruit-body-producing entomopathegenic species from different geographical regions in China. FEMS Microbiol Lett 230: 153–158. [DOI] [PubMed] [Google Scholar]
- 11. Chen J, Zhang W, Lu T (2006) Morphological and genetic characterization of a cultivated Cordyceps sinensis fungus and its polysaccharide component possessing antioxidant property in H22 tumor-bearing mice. Life Sci 78(23): 2742–2748. [DOI] [PubMed] [Google Scholar]
- 12. Leung PH, Zhang QX, Wu JY (2006) Mycelium cultivation, chemical composition and antitumour activity of a Tolypocladium sp. fungus isolated from wild Cordyceps sinensis . J Appl Microbiol 101(2): 275–283. [DOI] [PubMed] [Google Scholar]
- 13. Zhu J-S, Guo YL, Yao YS, Zhou YJ, Lu JH, et al. (2007) Maturation of Cordyceps sinensis associates with co-existence of Hirsutella sinensis and Paecilomyces hepiali DNA and dynamic changes in fungal competitive proliferation predominance and chemical profiles. J Fungal Res 5(4): 214–224. [Google Scholar]
- 14. Stensrud Ø, Schumacher T, Shalchian-Tabrizi K, Svegardenib IB, Kauserud H (2007) Accelerated nrDNA evolution and profound AT bias in the medicinal fungus Cordyceps sinensis . Mycol Res 111: 409–415. [DOI] [PubMed] [Google Scholar]
- 15. Yang JL, Xiao W, He HH, Zhu HX, Wang SF (2008) Molecular phylogenetic analysis of Paecilomyces hepiali and Cordyceps sinensis . Acta Pharmaceut Sinica 43(4): 421–426. [PubMed] [Google Scholar]
- 16. Xiao W, Yang JP, Zhu P, Cheng KD, He HX, et al. (2009) Non-support of species complex hypothesis of Cordyceps sinensis by targeted rDNA-ITS sequence analysis. Mycosystema 28(6): 724–730. [Google Scholar]
- 17. Zhang YJ, Sun BD, Zhang S, Wangmu, Liu XZ, et al. (2010a) Mycobiotal investigation of natural Ophiocordyceps sinensis based on culture-dependent investigation. Mycosistema 29(4): 518–527. [Google Scholar]
- 18. Zhang YJ, Zhang S, Wang M, Bai FY, Liu XZ (2010b) High Diversity of the Fungal Community Structure in Naturally-Occurring Ophiocordyceps sinensis. PLoS ONE 5(12): e15570 doi:10.1371/journal.pone.0015570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu J-S, Gao L, Li XH, Yao YS, Zhou YJ, et al. (2010) Maturational alterations of oppositely orientated rDNA and differential proliferations of CG:AT-biased genotypes of Cordyceps sinensis fungi and Paecilomyces hepiali in natural Cordyceps sinensis . Am J Biomed Sci 2(3): 217–238. [Google Scholar]
- 20. Gao L, Li XH, Zhao JQ, Lu JH, Zhu J-S (2011) Detection of multiple Ophiocordyceps sinensis mutants in premature stroma of Cordyceps sinensis by MassARRAY SNP MALDI-TOF mass spectrum genotyping. Beijing Da Xue Xue Bao 43(2): 259–266. [PubMed] [Google Scholar]
- 21.Yao YS, Zhou YJ, Gao L, Lu JH, Zhu J-S (2011) Dynamic alterations of the differential fungal expressions of Ophiocordyceps sinensis and its mutant genotypes in stroma and caterpillar during maturation of natural Cordyceps sinensis. J Fungal Res 9(1): : 37–49,53.
- 22. Gao L, Li XH, Zhao JQ, Lu JH, Zhao JG, et al. (2012) Maturation of Cordyceps sinensis associates with alterations of fungal expressions of multiple Ophiocordyceps sinensis mutants with transition and transversion point mutations in stroma of Cordyceps sinensis . Beijing Da Xue Xue Bao 44(3): 454–463. [PubMed] [Google Scholar]
- 23. Zhu J-S, Zhao JG, Gao L, Li XH, Zhao JQ, et al. (2012) Dynamically altered expressions of at least 6 Ophiocordyceps sinensis mutants in the stroma of Cordyceps sinensis . J Fungal Res 10(2): 100–112. [Google Scholar]
- 24. Xiao YY, Chen C, Dong JF (2011) Morphological observation of ascospores of Ophiocordyceps sinensis and its anamorph in growth process. J Anhui Agricult Univ 38(4): 587–591. [Google Scholar]
- 25. Hu X, Zhang YJ, Xiao GH, Zheng P, Xia YL, et al. (2013) Genome survey uncovers the secrets of sex and lifestyle in caterpillar fungus. Chin Sci Bull 58: 2846–2854. [Google Scholar]
- 26.Jin ZX, Yang SH (2005) Progresses and trends of Cordyceps sinensis studies. J Tianjin Med Univ 11(1): , 137–140.
- 27. Liang ZQ, Han YF, Liang JD, Dong X, Du W (2010) Issues of concern in the studies of Ophiocordyceps sinensis . Microbiol Chin 37(11): 1692–1697. [Google Scholar]
- 28.Ni LQ, Yao YS, Gao L, Wu ZM, Tan NZ, et al.. (2014) Density-weighted algorithms for similarity computation and cluster tree construction in the RAPD analysis of natural Cordyceps sinensis. Am J Biomed Sci 6(2): , 82–104.
- 29.Yao YS, Gao L, Li YL, Ma SL, Wu ZM, et al.. (2014) Amplicon density-weighted algorithms analyze dissimilarity and dynamic alterations of RAPD polymorphisms in the integrated micro-ecosystem Cordyceps sinensis. Beijing Da Xue Xue Bao 46(4): , 618–628. [PubMed]
- 30.Dai RQ, Lan JL, Chen WH, Li XM, Chen CT, et al.. (1989) Discovery of a new fungus Paecilomyces hepiali Chen & Dai. Acta Agriculturea Universitatis Pekinensis 15(2): , 221–224.
- 31. Ma SL, Li YL, Xu HF, Zhang ZH, Liu X (2010) Analyzing bacterial community in young Hepialus of intestinal tract of Cordyceps sinensis in Qinghai Province. Chin J Grassland 32(suppl.): 63–65. [Google Scholar]
- 32.Li YL, Yao YS, Ma SL, Xu HF, Li AP, et al.. (2014) Inoculation potency enhancement using fungal complexes isolated from the intestine of Hepialus armoricanus larvae. (ABS0144) The 10th International Mycological Congress, Bangkok, Thailand 3–8 August 2014.
- 33.Issaq HJ, Veenstra TD, Conrads TP, Felschow D (2002) The SELDI-TOF MS Approach to Proteomics: Protein Profiling and Biomarker Identification. Biochem Biophys Res Comm 292: , 587–592. [DOI] [PubMed]
- 34. Zeidan BA, Cutress RI, Hastie C, Mirnezami AH, Packham G, et al. (2009) SELDI-TOF MS Proteomics in Breast Cancer. Clin Proteom (2009) 5: 133–147. [Google Scholar]
- 35. Zhang P (2003) Advances in Cordyceps genus fungi research. J Biol 20(6): 43–45. [Google Scholar]
- 36.Shen NY, Zheng L, Zhang XC, Wei SL, Zhou ZR, et al.. (1983) Anamorph of Cordyceps sinensis (Berk) Sacc. in: Monograph for Cordyceps studies 1980-1985. Xining: Qinghai Acad Animal Sci Veterin Med pp 1–13.
- 37. Li ZZ, Huang B, Li CR, Fan MZ (2000) Molecular evidence for anamorph determination of Cordyceps sinensis (Berk.) Sacc. Mycosystema 9(1): 60–64. [Google Scholar]
- 38. Wu YX, Zhou DL, Yan D, Ren YS, Fang YL, et al. (2008) HPLC fingerprint analysis of Cordyceps and mycelium of cultured Cordyceps. Chin J Chin Materia Med 33(19): 2212–2214. [PubMed] [Google Scholar]
- 39.Yue DC, Feng X, Liu H, Bao TT (1995) Cordyceps sinensis, Chapter 4. In: Institute of Materia Medica (Ed.) Advanced Studies in Traditional Chinese Herbal Medicine, Vol. 1, Beijing Med. Univ. and Peking Union University Press, Beijing, pp. 91–113.
- 40.Li SP, Song ZH, Dong TTX, Ji ZN, Lo CK, et al.. (2004) Distinction of water-soluble constituents between natural and cultured Cordyceps by capillary electrophoresis. Phytomed 11: , 684–690. [DOI] [PubMed]
- 41. Hu Z, Xia FB, Wu XG, Wang Q, Xie JY, et al. (2004) New component analysis of essential oil from cultured Cordyceps sinensis . Edible Fungi Chin 23(5): 37–38. [Google Scholar]
- 42. Ye MQ, Hu Z, Fan Y, He L, Xia FB, et al. (2004) Purification and characterization of an acid deoxyribonuclease from the cultured mycelia of Cordyceps sinensis . J Biochem Mol Biol 37(4): 466–473. [DOI] [PubMed] [Google Scholar]
- 43. Wang S, Yang FQ, Feng K, Li DQ, Zhao J, et al. (2009) Simultaneous determination of nucleosides, myriocin, and carbohydrates in Cordyceps by HPLC coupled with diode array detection and evaporative light scattering detection. J Sep Sci 32: 4069–4076. [DOI] [PubMed] [Google Scholar]
- 44. Lu YY, Qiu XM, Jiang C, Liu SZ, Guo SW, et al. (2011) Analysis of nucleoside constituents in different parts of the artificial Cordyceps sinensis . Food Sci Technol 36(4): 250–256. [Google Scholar]
- 45. Yang ML, Kuo PC, Hwang TL, Wu TS (2011) Anti-inflammatory Principles from Cordyceps sinensis . J Nat Prod 74: 1996–2000. [DOI] [PubMed] [Google Scholar]
- 46. Wu YS, Zhou DL, Yan D, Ren YS, Fang YL, et al. (2008) HPLC fingerprint analysis of Cordyceps and mycelium of cultured Cordy. Chin J Chin Materia Medica 33(19): 2212–2214. [PubMed] [Google Scholar]
- 47. Lai YH, Ruan GP, Xie YL, Chen HA (2008) Study on HPLC Fingerprint Characteristic Analysis of Cordyceps sinensis and Its Similar Products. J Chin Med Mater 31(8): 1142–114. [PubMed] [Google Scholar]