Abstract
Erianthus arundinaceus is a valuable source of agronomic traits for sugarcane improvement such as ratoonability, biomass, vigor, tolerance to drought and water logging, as well as resistance to pests and disease. To investigate the introgression of the E. arundinaceus genome into sugarcane, five intergeneric F1 hybrids between S. officinarum and E. arundinaceus and 13 of their BC1 progeny were studied using the genomic in situ hybridization (GISH) technique. In doing so, we assessed the chromosome composition and chromosome transmission in these plants. All F1 hybrids were aneuploidy, containing either 28 or 29 E. arundinaceus chromosomes. The number of E. arundinaceus chromosomes in nine of the BC1 progeny was less than or equal to 29. Unexpectedly, the number of E. arundinaceus chromosomes in the other four BC1 progeny was above 29, which was more than in their F1 female parents. This is the first cytogenetic evidence for an unexpected inheritance pattern of E. arundinaceus chromosomes in sugarcane. We pointed to several mechanisms that may be involved in generating more than 2n gametes in the BC1 progeny. Furthermore, the implication of these results for sugarcane breeding programs was discussed.
Introduction
Sugarcane (Saccharum spp.) plays a pivotal role in world agriculture as a primary sugar-producing crop and has significant potential as a renewable bioenergy crop [1]. The genus Saccharum is comprised of six species: The two wild species are S. spontaneum and S. robustum, and the four cultivated species are S. officinarum, S. barberi, S. sinense and S. edule. S. officinarum (2n = 80) is known as the noble cane due to its high sugar content and thick and juicy culms. The wild species S. spontaneum (2n = 40–128; chromosome number varies) has very low sugar content but exhibits high vigor, profuse tillers and strong ratooning ability, as well as resistance to diseases and pests. Modern sugarcane cultivars are highly complex polyploid aneuploids and typically have 100–130 chromosomes derived from a combination of these two species [2].
During the early 20th century, interspecific hybridization was used to introgress desirable traits from wild species into sugarcane cultivars, and this practice led to substantial improvements in sugarcane agriculture [3]. In particular, interspecific hybridization between S. officinarum as the female parent and S. spontaneum as the male parent, followed by successive backcrosses of the hybrids to different clones of S. officinarum as the recurrent parent, significantly increased cane yields and resistance to biotic and abiotic stresses. In this hybridization strategy, F1 hybrids and plants in the first backcross generation (BC1) receive 2n gametes from female parent and n gametes from male parent, and plants in the second backcross generation (BC2) receives n gametes from both the female and male parents [4]. The purpose of the process termed nobilization, which refers to the crossing and backcrossing of intergenic hybrids to noble cane, was to retain high-sugar producing clones and to eliminate the negative effects of wild germplasm [5]. Over the past decades, much insight has been gained into the mechanisms underlying 2n gamete formation through the use of molecular genetic and cytological techniques. Using simple sequence repeat (SSR), amplified fragment length polymorphism (AFLP) and diversity arrays technology (DarT), Hermann et al. [6] provided molecular marker data that suggested the mechanism was second division restitution (SDR) or megaspore tetrad cell fusion (MTCF). Bielig et al. [7] provided cytological evidence that 2n male gamete formation was probably attributable to SDR. Nevertheless, the mechanisms underlying 2n gamete formation in sugarcane are still not fully understood.
Indeed, the occurrence of 2n gametes is not rare in the plant kingdom and it has been reported in many genera, including Brassica, Paspalum, Brachiaria, Citrus, Fragaria, Malus, Manihot, Medicago, Solanum, and Trifolium [8]–[11]. Several explanations for 2n gamete formation have been proposed, including pre-meiotic and post-meiotic genome doubling, and meiotic restitution. Among these possibilities, the majority of reports have identified a restitution of the meiotic cell cycle in several species [12]–[14], suggesting that it is the predominant mechanism of 2n gamete formation in plants. However, a small number of reports have documented pre-meiotic and post-meiotic genome duplications, indicating that these mechanisms of 2n gamete formation are quite rare. Three cytological processes can lead to 2n gamete formation during abnormal meiosis: first division restitution (FDR), second division restitution (SDR) and indeterminate meiotic restitution (IMR). In FDR, homologous chromosomes remain together when the nucleus fails to divide after telophase I, and after a normal second division, sister chromatids derived from each chromosome move to opposite poles. In SDR, normal separation of the homologous chromosomes at first division is followed by the absence of the second meiotic division and sister chromatids fail to migrate to opposite poles at second division. IMR has been best described in lily meiocytes, and simultaneously shows characteristics similar to both SDR and FDR within a single meiocyte [15]–[18].
Modern sugarcane cultivars have limited genetic diversity, due to the small number of progenitors used in the initial interspecific hybridizations during the process of nobilization [19]–[21]. This genetic bottleneck has impeded further sugarcane improvement for certain traits such as tolerance to biotic and abiotic stresses. Therefore, it is urgent to broaden the genetic base of sugarcane by introgressing favorable genes from closely related Erianthus, Miscanthus, Narenga and Sclerostachya genera [22].
Erianthus is one of the most closely related genera to Saccharum and has attracted the interest of sugarcane breeders worldwide. Erianthus arundinaceus (E. arundinaceus, 2n = 20, 40, 60) is one of eight species in the genus Erianthus [23], and it possesses valuable agronomic traits for sugarcane improvement such as high biomass, vigor, ratoonability, tolerance to drought and water logging, and resistance to pests and disease [23]–[28]. Favorable alleles can be introduced into modern sugarcane cultivars for yield and stability improvement, although the hybrid progeny is often sterile [26]. However, significant progress has been made to produce genuine F1 hybrids and to backcross the progeny successfully. Molecular markers and genomic in situ hybridization (GISH) techniques have been used to identify true intergeneric hybrids between Saccharum spp. and Erianthus spp. [23], [24], [26], [29]. According to histological staining character of root tips, Fukuhara et al. [30] concluded that F1 hybrids were successfully obtained from the intergeneric hybridization between Saccharum spp. hybrid and E. arundinaceus.
Using GISH, N. Piperidis et al. [29] reported that chromosome transmission was n+n in both F1 (S. officinarum × E. arundinaceus) and BC2 (BC1 × sugarcane cultivar) generations, but was 2n+n in the BC1 (F1 × sugarcane cultivar) cross. In similar crosses six F1 hybrids had fewer than 70 chromosomes and one had more than 70, indicating that all F1 crosses were aneuploidy [26]. In this report, we studied chromosome transmission in (E. arundinaceus × S. officinarum) hybrids by using GISH to determine the chromosome composition of two generations including five intergeneric F1 hybrids and 13 BC1 progeny.
Materials and Methods
Plant materials
The plant materials used in this study consisted of 18 clones derived from two generations of intergeneric hybrids (Table 1). The male parent of the F1 generation was either E. arundinaceus HN 92-77 (2n = 60) or HN 92–105 (2n = 60) from Hainan, China. S. officinarum Badila (2n = 80) was used as the female parent for the F1 generation. Female parents of the BC1 generation were YCE 95-41, YCE 96-40 and YCE 96-66, which were derived from crosses between Badila and HN 92-77 or HN 92–105. The male parent of the BC1 generation was CP 84–1198 (2n = 120), which is a commercial cultivar containing germplasm from S. officinarum, S. spontaneum, S. barberi and S. robustum without contribution from E. arundinaceus. F1 and BC1 plants were generated at the Hainan Sugarcane Breeding Station of Guangzhou Sugarcane Industry Research Institute. All clones were planted in the greenhouse at Fujian Agriculture and Forestry University.
Table 1. The intergeneric F1 hybrids and their BC1 progeny between Saccharum spp. and E. arundinaceus.
| Generation | Clones | Female parent | Male parent |
| F1 | YCE 96-66 | Badila | HN 92-105 |
| F1 | YCE 96-40 | Badila | HN 92-77 |
| F1 | YCE 96-43 | Badila | HN 92-77 |
| F1 | YCE 96-45 | Badila | HN 92-77 |
| F1 | YCE 95-41 | Badila | HN 92-77 |
| BC1 | YCE 01-33 | YCE 95-41 | CP 84-1198 |
| BC1 | YCE 01-46 | YCE 95-41 | CP 84-1198 |
| BC1 | YCE 01-48 | YCE 95-41 | CP 84-1198 |
| BC1 | YCE 01-36 | YCE 96-40 | CP 84-1198 |
| BC1 | YCE 01-92 | YCE 96-40 | CP 84-1198 |
| BC1 | YCE 01-99 | YCE 96-40 | CP 84-1198 |
| BC1 | YCE 01-102 | YCE 96-40 | CP 84-1198 |
| BC1 | YCE 01-105 | YCE 96-40 | CP 84-1198 |
| BC1 | YCE 01-116 | YCE 96-40 | CP 84-1198 |
| BC1 | YCE 01-134 | YCE 96-40 | CP 84-1198 |
| BC1 | YCE 01-63 | YCE 96-66 | CP 84-1198 |
| BC1 | YCE 01-61 | YCE 96-66 | CP 84-1198 |
| BC1 | YCE 01-69 | YCE 96-66 | CP 84-1198 |
Genomic in situ hybridization procedure
Chromosome preparation, chromosome spreading and GISH experiments were performed as described in D’hont et al. [24]. Genomic DNA from E. arundinaceus HN 92-77 and HN 92–105 was labeled with digoxigenin-11-dUTP (Roche) and genomic DNA from Badila and CP 84–1198 was labeled with biotin-16-dUTP (Roche) using the Nick Translation Kit (Roche). To detect signal from biotin-labeled probes, Avidin D, Rhodamine 600 (XRITC) and biotinylated anti-avidin antibody (Vector Laboratories, Burlingame, CA) were used. To detect signal from digoxigenin-labeled probes, sheep-anti-digoxin-FITC (Roche, Lewes, UK) and rabbit-anti-sheep-FITC (Roche, Lewes, UK) were used. Chromosomes were then counter stained using DAPI in Vectashield anti-fade solution Vectashield (Vector Laboratories, Burlingame, CA). The hybridization signals were observed on an AxioScope A1 Imager fluorescent microscope (Carl Zeiss, Gottingen, Germany). Images were captured digitally with an AxioCam MRc5 and AxioVision v.4.7 imaging software (Carl Zeiss, Gottingen, Germany).
Results and Discussion
Aneuploidy in F1 hybrids
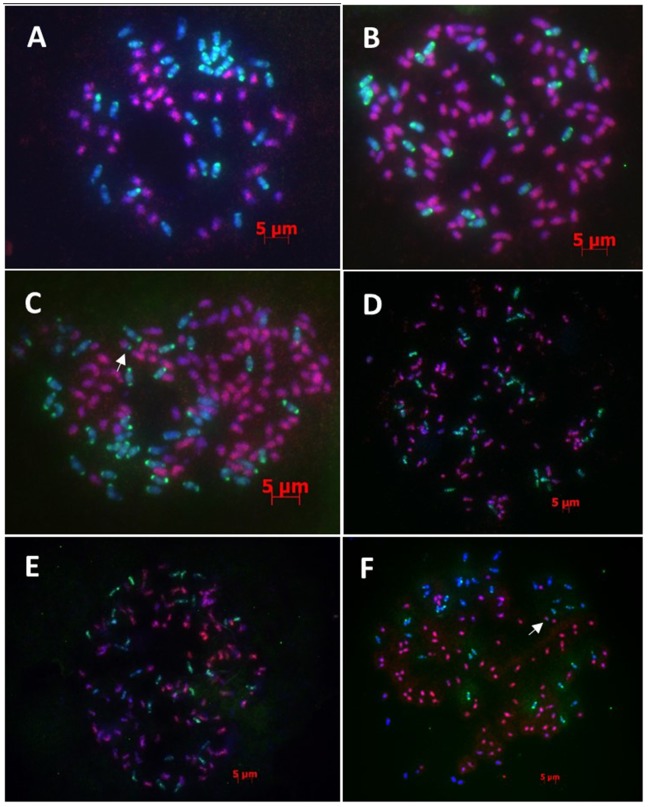
Five F1 hybrids analyzed by GISH were characterized by the presence of 68–69 chromosomes, consisting of 40 Saccharum-derived chromosomes and 28–29 E. arundinaceus-derived chromosomes (Figure 1A, Figure S1–S4 in File S1). Therefore, the five F1 hybrids were the products of n+n chromosome transmission (Table 2), and all hybrids were also aneuploid. These results are consistent with G. Piperidis’s studies [26]. Notably, the high rate of aneuploidy in F1 hybrids contributes to the production of unbalanced gametes, which might be associated with the high degree of sterility in F1 hybrids [31], [32]. Chromosomes inherited from divergent parents are often unable to pair with each other in meiosis [33]–[36], producing very few or no viable pollen grains [26], [37]. In order to obtain backcross generations, an adjustment in sugarcane breeding was implemented, involving F1 hybrids as female parents and CP 84-1198 as the male parent, although this does not conform perfectly to the fundamental principles of backcross breeding. Even though F1 hybrids were used as female parents, their fertility was still very low. Among the five F1 hybrids, only YCE 96-40, YCE 96-66 and YCE 95-41 as female parents generated several BC1 progeny by backcrossing with CP 84–1198. Attempts to backcross YCE 96-43 and YCE 96-45 to CP 84-1198 were not successful.
Figure 1. GISH analysis of the F1 hybrids and BC1 progeny.
Saccharum spp. chromosomes were visualized in red and E. arundinaceus chromosomes in green. (A) YCE 96-66 (F1): 29 chromosomes from E. arundinaceus and 40 chromosomes from Saccharum spp.; (B) YCE 01–102 (BC1): 22 chromosomes from E. arundinaceus and 96 chromosomes from Saccharum spp.; (C) YCE 01–36 (BC1): 36 chromosomes from E. arundinaceus, 96 chromosomes from Saccharum spp. and one terminally translocated chromosome; (D) YCE 01–61 (BC1): 31 chromosomes from E. arundinaceus and 85 chromosomes from Saccharum spp.; (E) YCE 01–69 (BC1): 31 chromosomes from E. arundinaceus and 88 chromosomes from Saccharum spp.; (F) YCE 01–92 (BC1): 35 chromosomes from E. arundinaceus, 95 chromosomes from Saccharum spp. and one terminally translocated chromosome. The arrowhead in Figure 1C and Figure 1F shows the translocated chromosome. Scale bars: 5 µm.
Table 2. Chromosome composition of F1 hybrids and BC1 progeny.
| Generation | Clones | Chromosome Composition | No. of cells observed | ||||||
| Modal number Range | Modal number Range | Modal number Range | Recombinants | ||||||
| 2n cell | Saccharum spp. | E. arundinaceus | |||||||
| F1 | YCE 95-41 | 68 | 68–70 | 40 | 40 | 28 | 28–30 | 0 | 4 |
| F1 | YCE 96-40 | 69 | 68–70 | 40 | 40 | 29 | 28–30 | 0 | 21 |
| F1 | YCE 96-43 | 69 | 68–70 | 40 | 40 | 29 | 28–30 | 0 | 4 |
| F1 | YCE 96-45 | 69 | 67–70 | 40 | 40 | 29 | 27–30 | 0 | 12 |
| F1 | YCE 96-66 | 69 | 67–70 | 40 | 40 | 29 | 27–30 | 0 | 22 |
| BC1 | YCE 01-33 | 120 | 118–121 | 93 | 91–93 | 27 | 26–28 | 0 | 5 |
| BC1 | YCE 01–46 | 125 | 122–127 | 96 | 95–97 | 29 | 28–29 | 0 | 6 |
| BC1 | YCE 01-48 | 120 | 117–121 | 93 | 93–94 | 27 | 26–29 | 0 | 15 |
| BC1 | YCE 01-63 | 125 | 123–126 | 97 | 95–98 | 28 | 26–28 | 0 | 8 |
| BC1 | YCE 01-99 | 118 | 117–120 | 95 | 95–97 | 23 | 21–23 | 0 | 4 |
| BC1 | YCE 01-102 | 118 | 115–119 | 96 | 94–97 | 22 | 19–24 | 0 | 23 |
| BC1 | YCE 01-105 | 117 | 115–119 | 94 | 93–96 | 23 | 22–23 | 0 | 12 |
| BC1 | YCE 01-116 | 122 | 119–124 | 94 | 92–95 | 28 | 26–29 | 0 | 10 |
| BC1 | YCE 01-134 | 121 | 120–122 | 93 | 93–95 | 28 | 26–29 | 0 | 8 |
| BC1 | YCE 01-36 | 134 | 130–136 | 96 | 95–97 | 36 | 35–36 | 1 | 10 |
| BC1 | YCE 01-92 | 130 | 129–132 | 95 | 94–96 | 35 | 34–36 | 1 | 8 |
| BC1 | YCE 01-61 | 116 | 114–118 | 85 | 84–85 | 31 | 30–31 | 0 | 3 |
| BC1 | YCE 01-69 | 119 | 115–120 | 88 | 87–90 | 31 | 29–32 | 0 | 8 |
Note: The modal number of chromosomes is presented for the sugarcane clones analysed, since small variation of chromosome counts can occur due to the loss or the overlapping of a few chromosomes from the preparation.
Unexpected inheritance pattern in BC1 progeny
GISH analysis of nine BC1 progeny revealed plants with a total chromosome complement ranging from 117 to 125 (Table 2), of which 93 to 97 chromosomes were derived from Saccharum and 22 to 29 chromosomes were derived from E. arundinaceus (Figure 1B, Figure S5–S12 in File S1). These results indicated that the nine BC1 progeny were products of 2n+n transmission. G. Piperidis et al. [26] and N. Piperidis et al. [29] reported similar results. GISH analysis of another four BC1 progeny revealed plants with a total chromosome complement ranging from 116 to 132, and evidence of an unusual mode of chromosome transmission (Table 2, Figure 1C–1F). In YCE 01–36, YCE 01–61, YCE 01–69 and YCE 01–92, 85 to 96 chromosomes were derived from Saccharum and 36, 31, 31 and 35 chromosomes were derived from E. arundinaceus, respectively. These results indicated that, in these four BC1 progeny, more than 29 E. arundinaceus-derived chromosomes (Table 2, Figure 1) were transmitted, which is a greater number than was detected in the F1 generation. To our knowledge, ours is the first report to document that the E. arundinaceus-derived chromosome number was above 29 in BC1 progeny. Within the plant kingdom, this unusual phenomenon has rarely been reported. It is especially noteworthy that four BC1 progeny out of 13 exhibited greater than 2n female-inherited chromosomes, suggesting that this newly discovered phenomenon can occur at relatively high frequency in sugarcane (above 30%). More importantly, the occurrence of more than 2n female gametes was detected in two diverse parental combinations rather than an individual case, suggesting that this phenomenon is not restricted to an individual plant. The results also suggest that the four BC1 progeny were the product of a new pattern of chromosome transmission. Interestingly, YCE 01–36 (Figure 1C) and YCE 01–92 (Figure 1F) were found to both have a terminally translocated chromosomes.
Possible mechanisms
The unexpected inheritance pattern that we observed in BC1 progeny is not in accordance with prevailing theories of chromosome transmission in hybrids. If meiosis occurs normally, four gametes are generated with different numbers of E. arundinaceus-derived chromosomes, due to the 29 E. arundinaceus chromosomes in F1 hybrids. As a result, two gametes are produced containing 14 E. arundinaceus chromosomes and the other two gametes contain 15 E. arundinaceus chromosomes. (Figure 2A). In FDR, two gametes are produced with 29 E. arundinaceus chromosomes (Figure 2B). In SDR, one gamete is produced with 28 E. arundinaceus chromosomes and a second contains 30 E. arundinaceus chromosomes (Figure 2C). SDR has been extensively reported in sugarcane [7], [38], [39]. In addition, Narayanaswami [40] discovered that 2n gametes originated from the fusion of the two innermost cells of the megaspore tetrad (megaspore tetrad cell fusion, MTCF). Post-meiotic restitution (PMR), in which chromosome doubling occurs after the second meiotic division, was observed by Bremer [38].
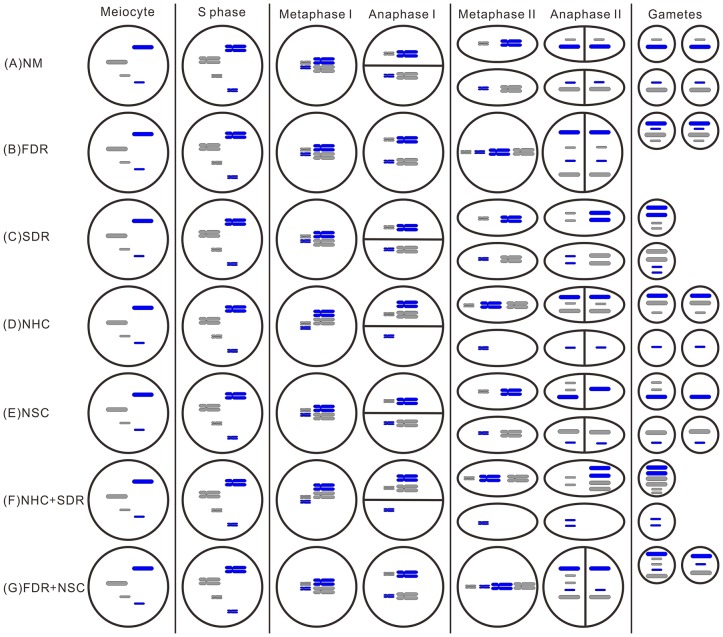
Figure 2. Schematic of seven meiosis scenarios with two pairs of homologous chromosomes.
(A) NM: normal meiosis; (B) FDR: first meiotic division restitution; (C) SDR: second meiotic division restitution; (D) NHC: nondisjunction of homologous chromosomes in first meiotic division; (E) NSC: nondisjunction of sister chromatids in second meiotic division; (F) NHC+SDR: nondisjunction of homologous chromosomes in first meiotic division and second division restitution; (G) FDR+NSC: first division restitution and nondisjunction of a chromosome with sister chromatids in the second meiotic division. For simplicity, recombination events are not illustrated in these meiosis schematics.
The normal separation of chromosomes in the first meiotic division or sister chromatids in the second meiotic division is called disjunction. Nondisjunction can occur in the first meiotic division (nondisjunction of homologous chromosomes; NHC) or second meiotic division (nondisjunction of sister chromatids; NSC). These distinct processes of nondisjunction create gametes with different numbers of chromosomes (Figure 2D, Figure 2E). In this study, we propose two possible mechanisms responsible for the formation of gametes with chromosome number greater than 2n. The first possibility (Model I) involves both NHC and SDR, which would generate two gametes with different even numbers of E. arundinaceus chromosomes after meiosis (Figure 2F; NHC + SDR). The second possibility (Model II) involves both FDR and NSC, which would generate two gametes with different odd or even numbers of E. arundinaceus chromosomes after meiosis (Figure 2G; FDR + NSC).
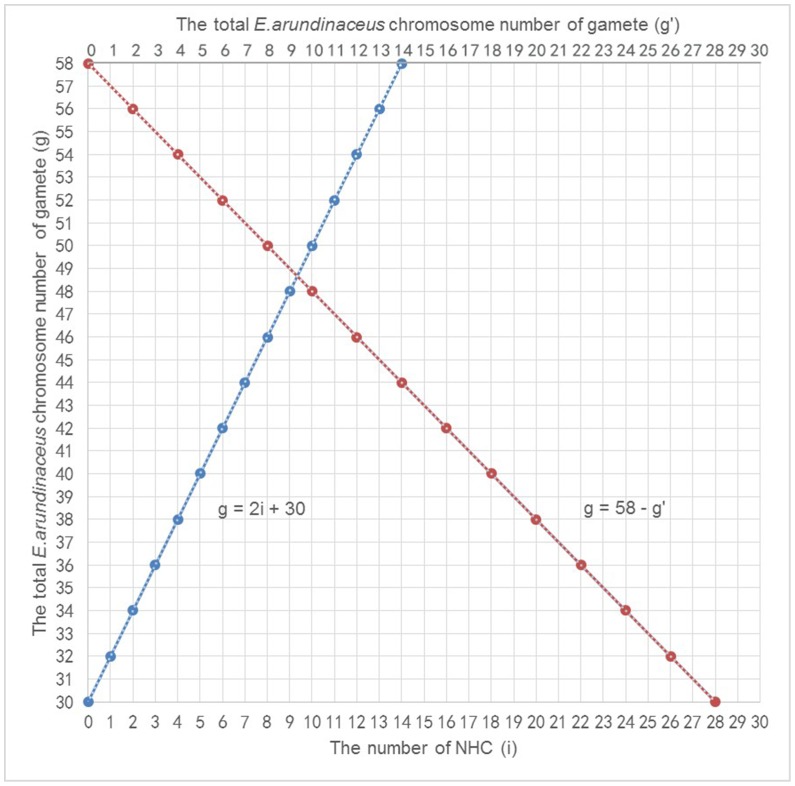
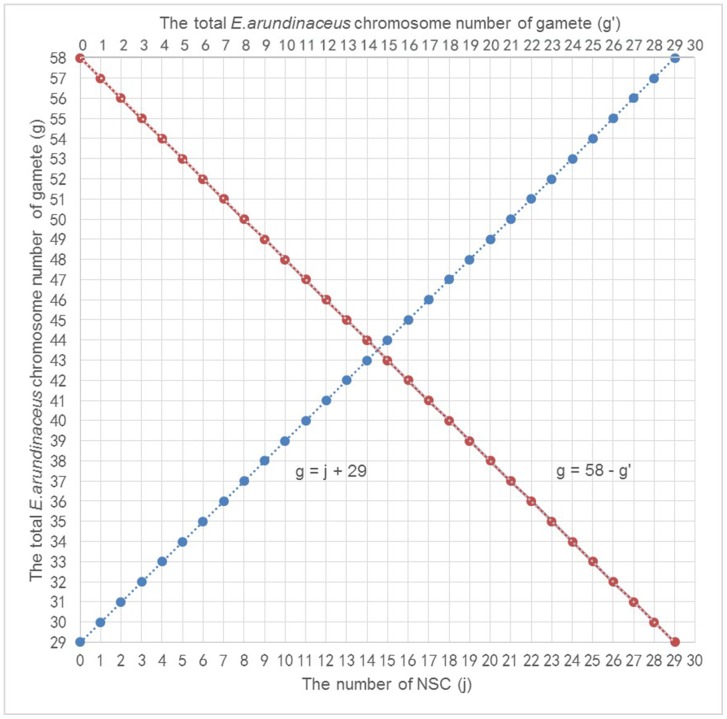
According to the results obtained from plants in the F1 generation, their meiocytes contained 29 E. arundinaceus chromosomes. During S phase of pre-meiotic interphase, all chromosomes are duplicated and each chromosome is comprised of two sister chromatids. Consequently, after meiosis, the total number of E. arundinaceus chromosomes in the F1 gametes should be 58. According to Model I, if there are i pair(s) of NHC in the first meiotic division, this yields a difference of 2(2i +1) E. arundinaceus chromosomes between the two gametes. According to Model II, if there are j chromosomes with NSC in the second meiotic division, this yields a difference of 2j E. arundinaceus chromosomes between the two gametes. Thus, the following two simultaneous linear equations are obtained:
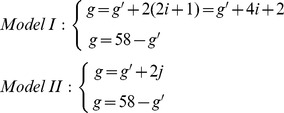 |
In these equations, g and g’ are the total number of E. arundinaceus chromosomes in each gamete after meiosis, and i and j are the number of NHC and NSC, respectively. From our experimental observations of 29 E. arundinaceus chromosomes in a meiocyte and 58 E. arundinaceus chromosomes in two gametes, we required that 0 ≤ g ≤ 58; 0 ≤ g’ ≤ 58; 0 ≤ i ≤ 14; and 0 ≤ j ≤ 29. The four variables g, g’, i and j were integral. After solving these simultaneous linear equations, we obtained two formulas:
Based on these formulas, graphs of these linear equations are shown in Figure 3 and Figure 4.
Figure 3. The relationship between NHC and the total E. arundinaceus chromosome number of gametes (blue curve), and the interrelationship of two gametes (red curve).
Note: g and g’ are the total number of E. arundinaceus chromosomes in each gamete after meiosis, i is the number of NHC.
Figure 4. The relationship between NSC and the total number of E. arundinaceus chromosomes in each gamete (blue curve), and the interrelationship of two gametes (red curve).
Note: g and g’ are the total number of E. arundinaceus chromosomes in each gamete after meiosis, j is the number of NSC.
In this study, eight different E. arundinaceus chromosome numbers were observed (22, 23, 27, 28, 29, 31, 35 and 36) in BC1 progeny. Due to the fact that we detected odd numbers of E. arundinaceus chromosomes in BC1 progeny, we speculate that Model II may be a more likely mechanism than Model I, but Model I cannot be ruled out as a mechanism occurring in plants that inherited even numbers of E. arundinaceus chromosomes. It is possible that both models are valid, suggesting that both mechanisms can occur.
Modern sugarcane cultivars are characterized by a high degree of inbreeding depression, so any increase in the heterozygosity of gametes may be beneficial to breeding efforts [6]. Depending on the specific mode of chromosome segregation, gametes can exhibit different degree of heterozygosity. During normal meiosis, crossing-over occurs between two non-sister chromatids. In FDR, 2n gametes always possess two non-sister chromatids and consequently maintain equivalent levels of parental heterozygosity and epistatic interactions. In SDR, sister chromatids do not separate and these gametes exhibit high levels of homozygosity. As a result, most parental heterozygosity and epistatic interactions are lost [41], [42]. The highest degree of heterogeneity is found in gametes originated through IMR, since these gametes result from a mixture of FDR and SDR [15]. In addition, when chromatids migrate to the same pole as in NHC and NSC, chromosomes are doubled. In NHC+SDR or FDR+NSC, some E. arundinaceus chromosomes would be doubled twice. This process likely creates a larger number of new multilocus allelic combinations and provides the opportunity to select the resulting germplasm for new, desirable traits.
Future directions
In order to understand the underlying mechanisms involved in generating the number of E. arundinaceus chromosomes in BC1 progeny, detailed cytological observations of female gametes and chromosomal dynamics in the embryo sac of F1 hybrids are needed. Although difficult to access, a more thorough understanding of the megagametophyte may result in possible applications for improving sugarcane through 2n gamete transmission.
Supporting Information
Supporting Information Figures. Figure S1. YCE 95-41(F1): 28 chromosomes from E. arundinaceus and 40 chromosomes from Saccharum spp. Figure S2. YCE 96-40(F1): 29 chromosomes from E. arundinaceus and 40 chromosomes from Saccharum spp. Figure S3. YCE 96-43(F1): 29 chromosomes from E. arundinaceus and 40 chromosomes from Saccharum spp. Figure S4. YCE 96-45(F1): 29 chromosomes from E. arundinaceus and 40 chromosomes from Saccharum spp. Figure S5. YCE 01–33 (BC1): 27 chromosomes from E. arundinaceus and 93 chromosomes from Saccharum spp. Figure S6. YCE 01–46 (BC1): 29 chromosomes from E. arundinaceus and 96 chromosomes from Saccharum spp. Figure S7. YCE 01–48 (BC1): 27 chromosomes from E. arundinaceus and 93 chromosomes from Saccharum spp. Figure S8. YCE 01–63 (BC1): 28 chromosomes from E. arundinaceus and 97 chromosomes from Saccharum spp. Figure S9. YCE 01–99 (BC1): 23 chromosomes from E. arundinaceus and 95 chromosomes from Saccharum spp. Figure S10. YCE 01–105 (BC1): 23 chromosomes from E. arundinaceus and 94 chromosomes from Saccharum spp. Figure S11. YCE 01–116 (BC1): 28 chromosomes from E. arundinaceus and 94 chromosomes from Saccharum spp. Figure S12. YCE 01–134 (BC1): 28 chromosomes from E. arundinaceus and 93 chromosomes from Saccharum spp.
(DOC)
Acknowledgments
We thank Dr. Jianping Wang for her assistance in English editing.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by National Natural Science Foundation of China (30671329), the earmarked fund for the Modern Agriculture Technology of China (CARS-20-1-5). This project is also supported by Guangxi Natural Science Foundation (2014GXNSFFA118002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Waclawovsky AJ, Sato PM, Lembke CG, Moore PH, Souza GM (2010) Sugarcane for bioenergy production: an assessment of yield and regulation of sucrose content. Plant Biotechnol J 8: 263–276. [DOI] [PubMed] [Google Scholar]
- 2. Jannoo N, Grivet L, Chantret N, Garsmeur O, Glaszmann JC, et al. (2007) Orthologous comparison in a gene-rich region among grasses reveals stability in the sugarcane polyploid genome. Plant J 50: 574–585. [DOI] [PubMed] [Google Scholar]
- 3. Alwala S, Kimbeng CA, Veremis JC, Gravois KA (2009) Identification of molecular markers associated with sugar-related traits in a Saccharum interspecific cross. Euphytica 167: 127–142. [Google Scholar]
- 4. Bremer G (1961) Problems in breeding and cytology of sugar cane. Euphytica 10: 59–78. [Google Scholar]
- 5. Roach B (1972) Nobilisation of sugarcane. Proc Int Soc Sugar Cane Technol 14: 206–216. [Google Scholar]
- 6. Hermann S, Aitken K, Jackson P, George A, Piperidis N, et al. (2012) Evidence for second division restitution as the basis for 2n+n maternal chromosome transmission in a sugarcane cross. Euphytica 187: 359–368. [Google Scholar]
- 7. Bielig LM, Mariani A, Berding N (2003) Cytological studies of 2n male gamete formation in sugarcane, Saccharum L. Euphytica. 133: 117–124. [Google Scholar]
- 8. Pfeiffer TW, Bingham ET (1983) Abnormal meiosis in alfalfa, Medicago sativa: cytology of 2n egg and 4n pollen formation. Can J Genet Cytol 25: 107–112. [Google Scholar]
- 9. Veilleux R (1985) Diploid and polyploid gametes in crop plants: mechanisms of formation and utilization in plant breeding. Plant Breed Rev 3: 253–288. [Google Scholar]
- 10. Werner JE, Peloquin S (1987) Frequency and mechanisms of 2n egg formation in haploid tuberosum-wild species F1 hybrids. Am J Bot 64: 641–654. [Google Scholar]
- 11. Souza AMd, Pagliarini MS, Carraro IM (1999) Abnormal spindles in second meiosis in canola (Brassica napus and Brassica campestris). Braz Arch Biol Technol 42: 47–52. [Google Scholar]
- 12. Bretagnolle F, Thompson JD (1995) Gametes with the somatic chromosome number: mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytol 129: 1–22. [DOI] [PubMed] [Google Scholar]
- 13. Pecrix Y, Rallo G, Folzer H, Cigna M, Gudin S, et al. (2011) Polyploidization mechanisms: temperature environment can induce diploid gamete formation in Rosa sp. J Exp Bot 62: 3587–3597. [DOI] [PubMed] [Google Scholar]
- 14. De Storme N, Copenhaver GP, Geelen D (2012) Production of Diploid Male Gametes in Arabidopsis by Cold-Induced Destabilization of Postmeiotic Radial Microtubule Arrays. Plant Physiol 160: 1808–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lim KB, Ramanna MS, de Jong JH, Jacobsen E, van Tuyl JM (2001) Indeterminate meiotic restitution (IMR): a novel type of meiotic nuclear restitution mechanism detected in interspecific lily hybrids by GISH. Theor Appl Genet 103: 219–230. [Google Scholar]
- 16. Barba-Gonzalez R, Ramanna MS, Visser RGF, Van Tuyl JM (2005) Intergenomic recombination in F1 lily hybrids (Lilium) and its significance for genetic variation in the BC1 progenies as revealed by GISH and FISH. Genome 48: 884–894. [DOI] [PubMed] [Google Scholar]
- 17. Zhou S, Ramanna M, Visser RG, van Tuyl JM (2008) Genome composition of triploid lily cultivars derived from sexual polyploidization of Longiflorum × Asiatic hybrids (Lilium). Euphytica 160: 207–215. [Google Scholar]
- 18. Khan N, Barba-Gonzalez R, Ramanna M, Arens P, Visser RG, et al. (2010) Relevance of unilateral and bilateral sexual polyploidization in relation to intergenomic recombination and introgression in Lilium species hybrids. Euphytica 171: 157–173. [Google Scholar]
- 19. Lu Y, D'Hont A, Walker D, Rao P, Feldmann P, et al. (1994) Relationships among ancestral species of sugarcane revealed with RFLP using single copy maize nuclear probes. Euphytica 78: 7–18. [Google Scholar]
- 20. Irvine J (1999) Saccharum species as horticultural classes. Theor Appl Genet 98: 186–194. [Google Scholar]
- 21. Edmé S, Glynn N, Comstock J (2006) Genetic segregation of microsatellite markers in Saccharum officinarum and S. spontaneum . Heredity 97: 366–375. [DOI] [PubMed] [Google Scholar]
- 22. Singh R, Singh R, Singh S, Sharma M (2011) Identification of sugarcane microsatellites associated to sugar content in sugarcane and transferability to other cereal genomes. Euphytica 182: 335–354. [Google Scholar]
- 23. Cai Q, Aitken KS, Fan YH, Piperidis G, Jackson P, et al. (2005) A preliminary assessment of the genetic relationship between Erianthus rockii and the “Saccharum complex” using microsatellite (SSR) and AFLP markers. Plant Sci 169: 976–984. [Google Scholar]
- 24. D'hont A, Rao P, Feldmann P, Grivet L, Islam-Faridi N, et al. (1995) Identification and characterisation of sugarcane intergeneric hybrids, Saccharum officinarum × Erianthus arundinaceus, with molecular markers and DNA in situ hybridisation. Theor Appl Genet 91: 320–326. [DOI] [PubMed] [Google Scholar]
- 25. Rott P, Mohamed I, Klett P, Soupa D, de Saint-Albin A, et al. (1997) Resistance to leaf scald disease is associated with limited colonization of sugarcane and wild relatives by Xanthomonas albilineans . Phytopathology 87: 1202–1213. [DOI] [PubMed] [Google Scholar]
- 26. Piperidis G, Christopher MJ, Carroll BJ, Berding N, D'Hont A (2000) Molecular contribution to selection of intergeneric hybrids between sugarcane and the wild species Erianthus arundinaceus . Genome 43: 1033–1037. [PubMed] [Google Scholar]
- 27. Ram B, Sreenivasan T, Sahi B, Singh N (2001) Introgression of low temperature tolerance and red rot resistance from Erianthus in sugarcane. Euphytica 122: 145–153. [Google Scholar]
- 28. Amalraj VA, Balasundaram N (2006) On the taxonomy of the members of ‘Saccharum complex’. Genet Resour Crop Evol 53: 35–41. [Google Scholar]
- 29. Piperidis N, Chen J, Deng H, Wang L, Jackson P, et al. (2010) GISH characterization of Erianthus arundinaceus chromosomes in three generations of sugarcane intergeneric hybrids. Genome 53: 331–336. [DOI] [PubMed] [Google Scholar]
- 30. Fukuhara S, Terajima Y, Irei S, Sakaigaichi T, Ujihara K, et al. (2013) Identification and characterization of intergeneric hybrid of commercial sugarcane (Saccharum spp. hybrid) and Erianthus arundinaceus (Retz.) Jeswiet. Euphytica 189: 321–327. [Google Scholar]
- 31. Phillips LL (1962) Segregation in New Allopolyploids of Gossypium.4. Segregation In New World X Asiatic and New World X Wild American Hexaploids. Am J Bot 49: 51–57. [Google Scholar]
- 32. Phillips LL (1964) Segregation in New Allopolyploids of Gossypium.V. Multivalent Formation in New World X Asiatic+New World X Wild American Hexaploids. Am J Bot 51: 324–329. [Google Scholar]
- 33. Ellstrand NC, Schierenbeck KA (2000) Hybridization as a stimulus for the evolution of invasiveness in plants? Proc Natl Acad Sci USA 97: 7043–7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Birchler JA, Bhadra U, Bhadra MP, Auger DL (2001) Dosage-dependent gene regulation in multicellular eukaryotes: implications for dosage compensation, aneuploid syndromes, and quantitative traits. Dev Biol 234: 275–288. [DOI] [PubMed] [Google Scholar]
- 35. Birchler JA, Yao H, Chudalayandi S (2007) Biological consequences of dosage dependent gene regulatory systems. Biochim Biophys Acta 1769: 422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goff SA (2011) A unifying theory for general multigenic heterosis: energy efficiency, protein metabolism, and implications for molecular breeding. New Phytol 189: 923–937. [DOI] [PubMed] [Google Scholar]
- 37. Govindaraj P, Balamurugan A, Natarajan US (2012) Identification of intergeneric hybrids between Erianthus arundinaceus and Saccharum spontaneum through STMS markers. Int Sugar J 114: 350–356. [Google Scholar]
- 38. Bremer G (1959) Increase of Chromosome number in Species-hybrids of Saccharum in Relation to the Embryo-sac Development. Bibliog Genet 18: 1–99. [Google Scholar]
- 39. Ramanna MS, Jacobsen E (2003) Relevance of sexual polyploidization for crop improvement-A review. Euphytica 133: 3–8. [Google Scholar]
- 40. Narayanaswami S (1940) Megasporogenesis and the origin of triploids in Saccharum . Indian J Agric Sci 10: 534. [Google Scholar]
- 41. Cai X, Xu SS (2007) Meiosis-driven genome variation in plants. Curr Genomics 8: 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. De Storme N, Geelen D (2011) The Arabidopsis mutant jason produces unreduced first division restitution male gametes through a parallel/fused spindle mechanism in meiosis II. Plant Physiol 155: 1403–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figures. Figure S1. YCE 95-41(F1): 28 chromosomes from E. arundinaceus and 40 chromosomes from Saccharum spp. Figure S2. YCE 96-40(F1): 29 chromosomes from E. arundinaceus and 40 chromosomes from Saccharum spp. Figure S3. YCE 96-43(F1): 29 chromosomes from E. arundinaceus and 40 chromosomes from Saccharum spp. Figure S4. YCE 96-45(F1): 29 chromosomes from E. arundinaceus and 40 chromosomes from Saccharum spp. Figure S5. YCE 01–33 (BC1): 27 chromosomes from E. arundinaceus and 93 chromosomes from Saccharum spp. Figure S6. YCE 01–46 (BC1): 29 chromosomes from E. arundinaceus and 96 chromosomes from Saccharum spp. Figure S7. YCE 01–48 (BC1): 27 chromosomes from E. arundinaceus and 93 chromosomes from Saccharum spp. Figure S8. YCE 01–63 (BC1): 28 chromosomes from E. arundinaceus and 97 chromosomes from Saccharum spp. Figure S9. YCE 01–99 (BC1): 23 chromosomes from E. arundinaceus and 95 chromosomes from Saccharum spp. Figure S10. YCE 01–105 (BC1): 23 chromosomes from E. arundinaceus and 94 chromosomes from Saccharum spp. Figure S11. YCE 01–116 (BC1): 28 chromosomes from E. arundinaceus and 94 chromosomes from Saccharum spp. Figure S12. YCE 01–134 (BC1): 28 chromosomes from E. arundinaceus and 93 chromosomes from Saccharum spp.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.