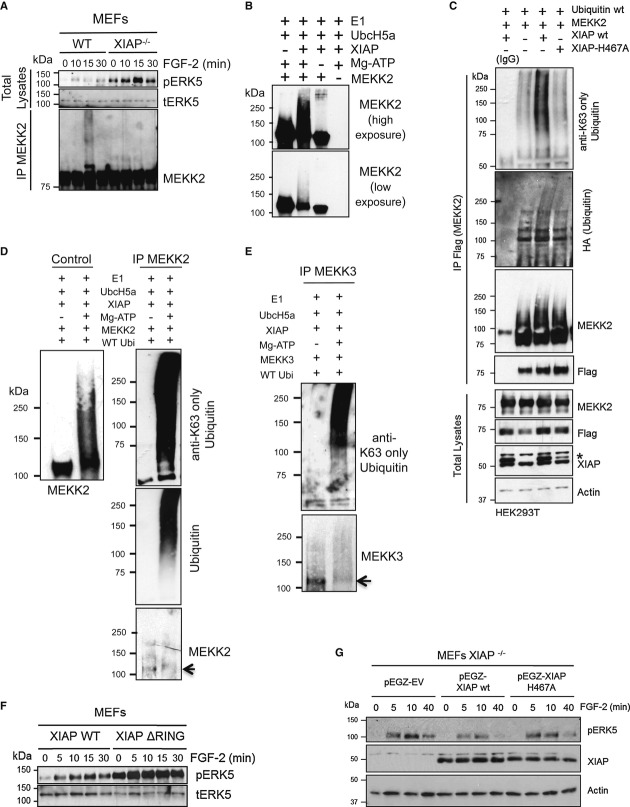
Figure 3. XIAP promotes K63-linked ubiquitin chain formation on MEKK2 and MEKK3 in a RING-dependent manner.
A XIAP mediates ubiquitination of MEKK2 after ERK5 activation in vivo. WT and XIAP−/− MEFs were stimulated with 25 ng/ml of FGF-2 for the indicated time points and MEKK2 was immunoprecipitated. Total lysates and IP fraction were analyzed by Western blotting.
B XIAP promotes MEKK2 ubiquitination in vitro. In vitro ubiquitination of purified MEKK2 by XIAP was performed and analyzed by Western blotting using MEKK2 antibody.
C K63-ubiquitination of MEKK2 is dependent of XIAP-E3 ligase activity. Flag-MEKK2 and HA-Ubiquitin were transfected in HEK293T cells with myc-EV, XIAP, or XIAP-H467A, an XIAP RING mutant. MEKK2 was immunoprecipitated using Flag antibody, and ubiquitination was checked using Western blot analysis with HA (ubiquitin) and K63-linkage-specific antibodies. *denotes overexpressed XIAP.
D, E XIAP promotes MEKK2 and MEKK3 K63-specific ubiquitination in vitro. MEKK2 (D) and MEKK3 (E) were subjected to in vitro ubiquitination, and MEKK2 or MEKK3, respectively, was immunoprecipitated from the samples. Western blot analysis was performed using K63-linkage-specific antibody.
F ERK5 phosphorylation is dependent on XIAP RING domain. WT and XIAP ΔRING knock-in MEFs were stimulated with 25 ng/ml of FGF-2 for indicated time points, and phosphorylation of ERK5 was detected by immunoblots.
G XIAP−/− MEFs were stably reconstituted with pEGZ-Flag EV, pEGZ-Flag-XIAP wt, or pEGZ-Flag-XIAP H467A were stimulated with 25 ng/ml of FGF-2 for the indicated time points. The activation of ERK5 was monitored by immunoblots.
Source data are available online for this figure.