Figure 4. XIAP-mediated MEKK2/3 ubiquitination directly impedes ERK5 activation.
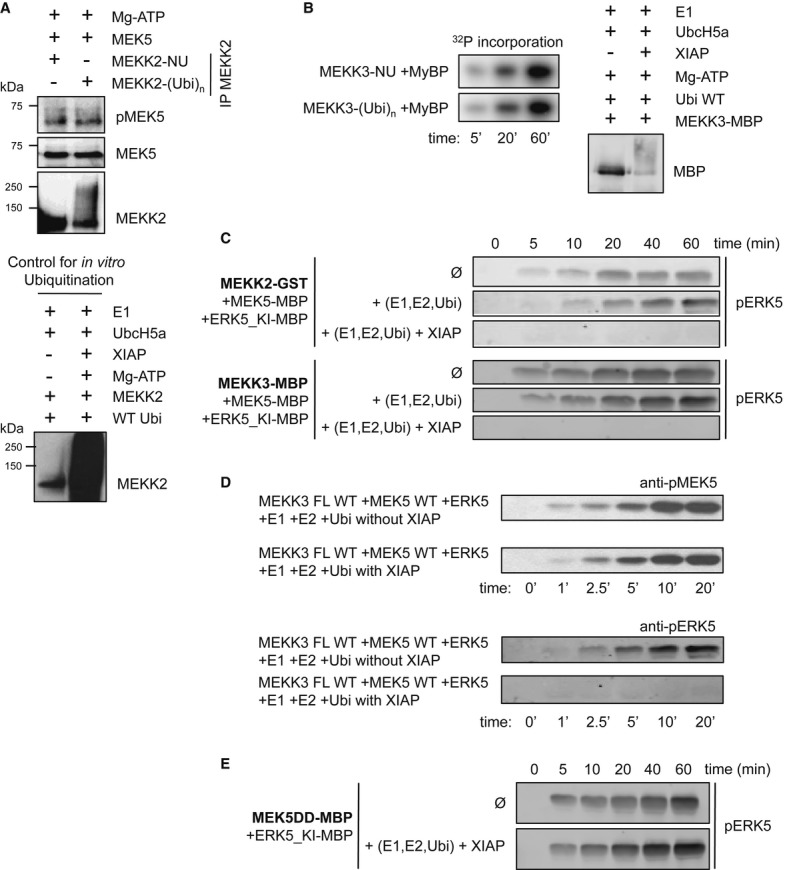
- MEKK2 activity on MEK5 is not affected by ubiquitination. In vitro ubiquitination of MEKK2 was performed (lower panel) and then was immunoprecipitated out from the ubiquitination mix. Beads with non-ubiquitinated (-NU) and ubiquitinated (-(Ubi)n) MEKK2 were employed for kinase assay with recombinant MEK5 as substrate (higher panel). Western blotting with phospho-specific MEK5 antibody was used as readout for the experiment.
- MEKK3 activity on a general kinase substrate (myelin basic protein, MyBP) is not affected by ubiquitination. In vitro ubiquitination of MEKK3-MBP was performed as described in Materials and Methods and checked by Western blot (right panel). Non-ubiquitinated (-NU) and ubiquitinated (-(Ubi)n) MEKK3 was incubated with 20 μM MyBP in the presence of ATP(γ-32P) and MyBP phosphorylation was monitored by phosphorimaging (left panel).
- XIAP-mediated ubiquitination interferes with ERK5 phosphorylation in the reconstituted MEKK2-MEK5-ERK5 MAPK module (higher panel). Similar results were found in MEKK3-MEK5-ERK5 reconstitution experiments (lower panel). ERK5 phosphorylation by MEK5 was monitored by Western blots (mutationally inactivated ERK5 was used in all experiments, ERK5_KI D182A). MEKK2 and MEKK3 were ubiquitinated as described for (B) and then an in vitro kinase assay was started by adding additional 0.5 mM ATP and recombinantly expressed and purified MEK5 and ERK5 (1 μM and 5 μM, respectively).
- XIAP-mediated ubiquitination interferes with ERK5 phosphorylation in the reconstituted MEKK3-MEK5-ERK5 MAPK module without any effect on MEK5 activation. MEK5 and ERK5 phosphorylation were monitored by Western blots (mutationally inactivated ERK5 was used in all kinase assays, ERK5_KI D182A). MEKK3 was ubiquitinated and then an in vitro kinase assay was performed as described for (C).
- Ubiquitination does not interfere with MEK5 mediated phosphorylation of ERK5, as the constitutively activated form of MEK5 (MEK5DD) activates ERK5 independent of the presence of XIAP (in all assays, the following concentrations were employed: MEKK2/3: 0.2 μM, MKK5: 1 μM, ERK5: 5 μM).
Source data are available online for this figure.
