Figure 1. Binding of substrates in Mhp1.
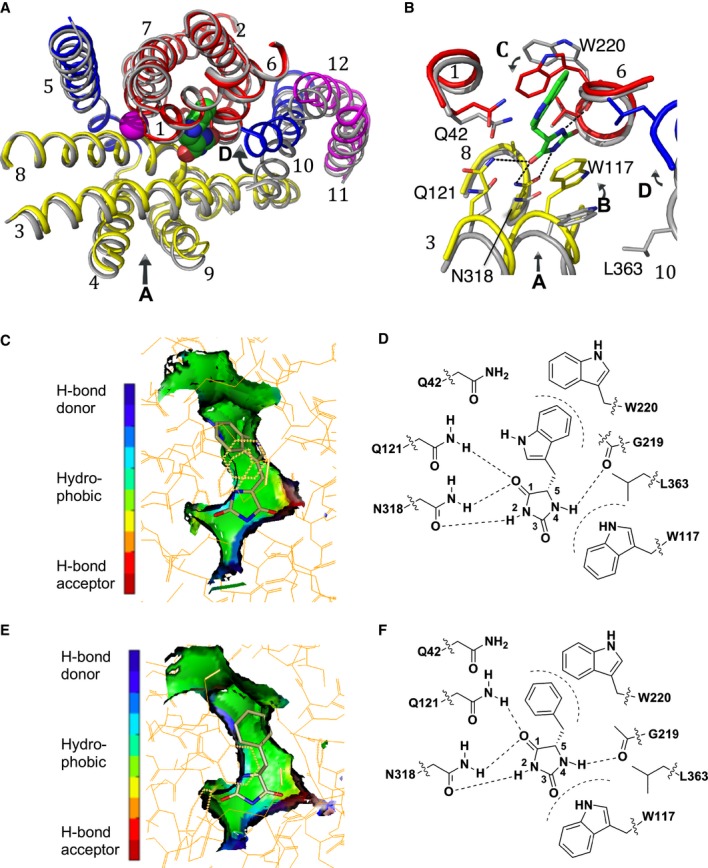
A, B Superposition of the outward-open structure (PDB code 2JLN) onto the IMH-bound structure, optimised using the bundle helices. The IMH structure is shown with the bundle in red, the hash motif in yellow, TMHs 5 and 10 in blue and the C-terminal helices in magenta. The outward-open structure is shown in grey. The L-IMH (green spheres) and sodium ion (magenta) bind between the hash and bundle motifs. (A) shows an overview of all helices and (B) a close up. The arrows show the main conformational changes that occur upon L-IMH binding. Arrow A: the hash motif rotates towards the bundle with the C-terminal helices partially following. Arrows B and C: Trp117 and Trp220 rotate towards the hydantoin moiety and the 5-indole substituent, respectively, of L-IMH. Arrow D: TMH10 flexes and packs over the IMH.
C The extended form of L-IMH in the binding site illustrated using the SPROUT format (Materials and Methods and Supplementary Methods) to show the indole moiety in a hydrophobic pocket (green).
D Schematic of interactions made between L-IMH and the protein. Possible hydrogen bonds are indicated by straight dashed lines and hydrophobic interactions by curved dashed lines.
E The extended form of L-BH is oriented similarly to L-IMH with its benzyl moiety in the hydrophobic pocket.
F Schematic of the interactions made by L-BH with Mhp1.
Data information: In (C and E) green represents regions where a hydrophobic interaction can be made, blue represents regions containing hydrogen-bond donor atoms, and red represents regions containing hydrogen-bond acceptor atoms.
