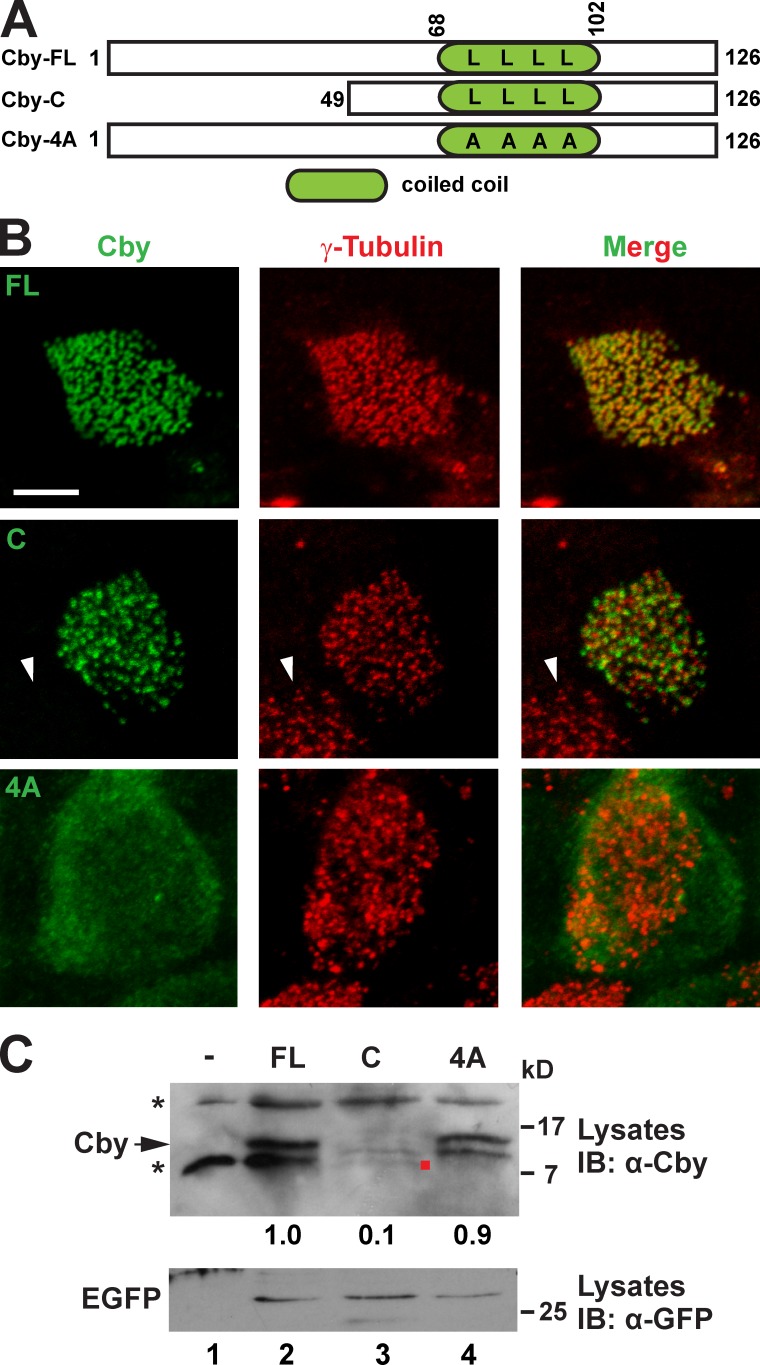
Figure 2.
The C-terminal coiled-coil motif is essential for Cby basal body localization. (A) Schematic depiction of human Cby constructs used in this study. The numbers indicate amino acid positions. Four leucines at positions 77, 84, 91, and 98 crucial for Cby homodimerization are shown. The dimerization-defective Cby-4A mutant contains alanine substitutions for all four leucine residues. (B) CbyKO MTECs were infected with lentiviruses encoding human full-length Cby (FL), Cby-C containing a leucine zipper coiled-coil motif, or Cby-4A mutant and colabeled with Cby and γ-tubulin antibodies at ALId14. Note that no Cby fluorescence was seen in noninfected ciliated cells (arrowheads). Bar, 5 µm. (C) Western blotting for expression of Cby mutants. Cell lysates were prepared from uninfected or infected CbyKO MTECs at ALId14 and probed with the Cby antibody. Note that Cby-C was detected at low levels (red dot). The asterisks indicate nonspecific bands present in uninfected cell lysates. The lentiviral constructs expressed EGFP from internal ribosome entry site, and similar transduction rates were verified by Western blotting with the GFP antibody. The band intensity of Cby proteins was quantified and normalized to that of EGFP. The normalized value for FL-Cby was set as 1. IB, immunoblot.