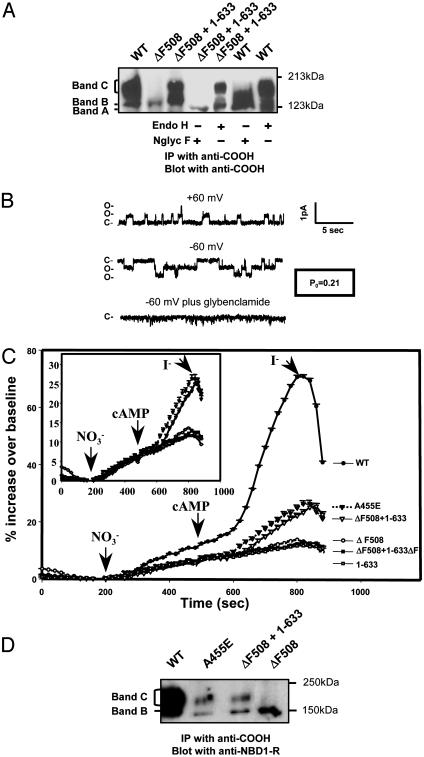
Fig. 3.
Rescued ΔF508-CFTR is fully glycosylated and exhibits chloride channel activity at the cell surface. (A) Transcomplemented ΔF508-CFTR was digested by N-glycosidase F (Nglyc F) but not by endoglycosidase H (endo H). Band A represents the unglycosylated protein. (B) CFTR channel activity in inside-out membrane patch excised from a COS-7 cell 24 h after cotransfection with ΔF508-CFTR and fragment 1–633. This patch had two detectable channels with linear current–voltage behavior, single-channel conductances of 6–8 pS, and sensitivity to 250 μM glibenclamide, all features of CFTR channels. Po was estimated from a 5-min record by using pclamp 8 software (Axon Instruments), assuming two active channels in the patch. (C) Rescued ΔF508-CFTR exhibits macroscopic transport activity comparable to that of A455E-CFTR, a partial processing mutant associated with mild disease. Macroscopic halide permeability was monitored for COS-7 cells 24 h after transfection by using a fluorescence-based assay (SPQ assay) and conditions identical to those described in Methods (18). Each data point represents the mean change in fluorescence for 9–42 responding cells (i.e., cells that exhibit an increase in fluorescence in response to a cAMP mixture) from at least three different coverslips. Error bars (SEMs) are smaller than the symbols. (Inset) Enlarged view of the permeability responses of cells transfected with A455E (▾) or cotransfected with fragment 1–633 and ΔF508-CFTR (▿) to the cAMP mixture. (D) Biochemical analysis of the band C forms of transcomplemented ΔF508-CFTR and A455E-CFTR in transfected COS-7 cells. Cells were analyzed for protein expression 48 h after transfection as described in Methods.