Abstract
Study Objectives:
Determine the effects of high versus moderate workload on sleep physiology and neurobehavioral measures, during sleep restriction (SR) and no sleep restriction (NSR) conditions.
Design:
Ten-night experiment involving cognitive workload and SR manipulations.
Setting:
Controlled laboratory environment.
Participants:
Sixty-three healthy adults (mean ± standard deviation: 33.2 ± 8.7 y; 29 females), age 22–50 y.
Interventions:
Following three baseline 8 h time in bed (TIB) nights, subjects were randomized to one of four conditions: high cognitive workload (HW) + SR; moderate cognitive workload (MW) + SR; HW + NSR; or MW + NSR. SR entailed 5 consecutive nights at 4 h TIB; NSR entailed 5 consecutive nights at 8 h TIB. Subjects received three workload test sessions/day consisting of 15-min preworkload assessments, followed by a 60-min (MW) or 120-min (HW) workload manipulation comprised of visually based cognitive tasks, and concluding with 15-min of postworkload assessments. Experimental nights were followed by two 8-h TIB recovery sleep nights. Polysomnography was collected on baseline night 3, experimental nights 1, 4, and 5, and recovery night 1 using three channels (central, frontal, occipital [C3, Fz, O2]).
Measurements and Results:
High workload, regardless of sleep duration, increased subjective fatigue and sleepiness (all P < 0.05). In contrast, sleep restriction produced cumulative increases in Psychomotor Vigilance Test (PVT) lapses, fatigue, and sleepiness and decreases in PVT response speed and Maintenance of Wakefulness Test (MWT) sleep onset latencies (all P < 0.05). High workload produced longer sleep onset latencies (P < 0.05, d = 0.63) and less wake after sleep onset (P < 0.05, d = 0.64) than moderate workload. Slow-wave energy—the putative marker of sleep homeostasis—was higher at O2 than C3 only in the HW + SR condition (P < 0.05).
Conclusions:
High cognitive workload delayed sleep onset, but it also promoted sleep homeostatic responses by increasing subjective fatigue and sleepiness, and producing a global sleep homeostatic response by reducing wake after sleep onset. When combined with sleep restriction, high workload increased local (occipital) sleep homeostasis, suggesting a use-dependent sleep response to visual work. We conclude that sleep restriction and cognitive workload interact to influence sleep homeostasis.
Citation:
Goel N, Abe T, Braun ME, Dinges DF. Cognitive workload and sleep restriction interact to influence sleep homeostatic responses. SLEEP 2014;37(11):1745-1756.
Keywords: alertness, cognitive workload, fatigue, polysomnography, psychomotor vigilance task, sleep homeostasis, sleepiness, sleep restriction, slow-wave activity, slow-wave energy
INTRODUCTION
Sleep loss is a well-documented, critical factor involved in fatigue-related accidents and human errors.1,2 Although fatigue results from the physiological consequences of inadequate sleep, prolonged wakefulness, and being awake at a circadian time when the brain is programmed to sleep,3–6 it can also occur because of excess cognitive or physical workload including high work demands or long work duration.7,8 Indeed, increased workload has long been recognized as a risk factor for fatigue, accidents, and injuries in the medical and other applied and occupational settings.7–15 To ensure successful assignment completion, workers must maintain a high level of performance in the face of demanding (high) workloads and work-rest schedules that result in chronic sleep restriction, both of which can contribute to fatigue and performance deficits.
Epidemiological studies have also investigated the relationship between workload and sleep difficulties.9,16,17 Åkerstedt et al. demonstrated that high work demand, but not longer duration of work, was associated with sleep disturbance.9,16 A longitudinal study using a French cohort demonstrated that sleep difficulties were associated with both highly demanding work and longer work duration.17 Although these epidemiological studies have shown a relationship between work demand and/or work duration and sleep disturbance, the causal effects of these factors on sleep disturbance remains unclear, because their respective contributions cannot be separated in these studies. A few experimental studies have investigated the effects of cognitive workload on polysomnographic (PSG) measures and slow-wave energy (SWE) as well as slow-wave activity (SWA)—a putative marker of sleep homeostasis18—but the results have been inconsistent.19–23 Some studies, but not others,20,21 showed delayed sleep onset latency (SOL) after cognitive tasks.19,22,23 In addition, some studies have failed to find SWE/SWA changes21–23 but other studies have found decreased SWE/SWA after cognitive tasks.20 All of these studies, however, limited the duration of cognitive workload to a single task or 1 day, and did not assess chronic effects—the latter is more representative of many work settings.
Prior studies also have only used derivations (channels) over the central regions20–23 even though several studies have demonstrated that increases in SWA occur locally in brain regions actively used during wakefulness by various types of tasks or stimulation. For example, SWA increased in the left central area over the somatosensory cortex following stimulation of the right hand24; SWA increased in the right parietal area following a learning task25; and SWA increased in the left central area over the motor cortex following repetitive transcranial magnetic stimulation to this region.26 Therefore, to thoroughly determine the effects of cognitive workload after waking activity on local sleep homeostasis, multiple electroencephalographic (EEG) channel analyses are required.
Independent of workload, other studies have investigated the effects of sleep restriction (SR) on sleep physiology,4–6,27–34 reporting increases in SWE and SWA during consecutive nights of SR.6,27,28,30–34 Although sleep physiology reflects the interaction of homeostatic and circadian processes,35 sleep may also reflect use-dependent processes occurring during sleep deprivation.32,36–40 Previous studies showed increases in SWA in frontal areas after total sleep deprivation in humans36–38 and during chronic sleep restriction (5 nights, 4 h time in bed [TIB]) in rats.32 Because the frontal area is among the most active brain regions during wakefulness, researchers have hypothesized that homeostatic sleep pressure locally accumulates during sleep deprivation, resulting in increased SWA during non-rapid eye movement (NREM) sleep in this brain region.36–38 Although such a hypothesis may be correct, these prior studies did not report using cognitive testing during wakefulness and cannot rule out the possibility of local activation in other brain regions.
We reported previously that high cognitive workload (HW) using visual tasks during 36 h of total sleep deprivation increased NREM SWA in the occipital (O2) EEG derivation during recovery (R) sleep compared to moderate cognitive workload (MW)39 and produced greater deficits in behavioral attention, cognitive speed, and perceived effort and sleepiness.41 Recently, another study found that different brain regions showed local SWA increases during NREM sleep in the same subjects after audiobook listening (produced activation in the left frontotemporal area) or driving simulation (produced activation in the occipitoparietal network) during R sleep following total sleep deprivation.40 Although our laboratory has reported SWA and SWE changes in frontal (Fz) central (C3), and O2 derivations as a function of various genetic polymorphisms during chronic sleep restriction,31,33,34 no study to date has systematically compared SWA and SWE directly across channels and as a function of manipulated cognitive workload during chronic SR in humans.
We designed the first systematic controlled laboratory experiment to investigate how workload and its combination with consecutive days of SR affects sleep physiology and neurobehavioral outcomes including fatigue, sleepiness, and attention. We hypothesized that HW would induce difficulties in sleeping, especially in the no sleep restriction (NSR) condition where there is no additional pressure for sleep from SR. Moreover, we hypothesized that (1) sleep restriction would produce a greater increase in SWA/SWE in the Fz region relative to other channels; (2) HW would produce a greater increase in SWA/SWE in the O2 region relative to other channels, because of local activation from visually based cognitive tasks; (3) the greatest increases in the Fz and O2 regions would occur in the HW + SR condition; and (4) HW, compared with MW would potentiate cumulative impairments in response speed and lapses in attention, increased subjective fatigue and sleepiness, and decreased physiological alertness resulting from sleep loss.
METHODS
Subjects
A total of n = 63 adults (29 women and 34 men, age 22-50 y, 33.2 ± 8.7 y) of various races (37 African Americans, 19 Caucasians, 7 biracial or multiracial individuals) were randomized to one of four different conditions: (1) HW + SR (n = 18); (2) MW + SR (n = 18); (3) HW + no sleep restriction (NSR) (n = 16); or (4) MW + NSR (n = 11). We did not include a no-workload condition in our design, because we wanted to simulate realistic work-rest schedules. All subjects were included in the neurobehavioral analyses. For the sleep analyses, subjects with missing or artifact-ridden PSG data were not included, yielding a total of n = 58 subjects (HW + SR: n = 16, 34.7 ± 8.4 y, 7 females; MW + SR: n = 17, 29.1 ± 7.3 y, 9 females; HW + NSR: n = 14, 34.1 ± 9.2 y, 6 females; MW + NSR: n = 11, 35.7 ± 8.3 y, 4 females).
In order to be eligible for study participation, subjects met the following inclusionary criteria: age range from 22–50 y; physically and psychologically healthy, as assessed by physical examination and history; no clinically significant abnormalities in blood chemistry; drug-free urine samples; good habitual sleep, between 6.5–8.5 h daily duration with regular bedtimes, and wake up times between 06:00–09:00 (verified by sleep logs and wrist actigraphy for at least 1 w before study entry); absence of extreme morningness/eveningness, as assessed by questionnaire42; absence of sleep or circadian disorders, as assessed by questionnaire43 and PSG; no history of psychiatric illness and no previous adverse neuropsychiatric reaction to sleep deprivation; no history of alcohol or drug abuse; and no current use of medical or drug treatments (excluding oral contraceptives). The study was approved by the Institutional Review Board of the University of Pennsylvania and all subjects received compensation for participation.
Procedures
The experiment took place in a controlled environment in the Sleep and Chronobiology Laboratory at the Hospital of the University of Pennsylvania. Ambient light was fixed at less than 50 lux during scheduled wakefulness, and less than 1 lux (darkness) during scheduled sleep, and did not differ across the four experimental conditions. Ambient temperature was maintained at 22 ± 1°C. Subjects were continuously monitored by trained staff. Subjects received three standardized meals per day, plus an optional healthy evening snack. Intake of caffeine, turkey, bananas, alcohol, or tobacco was prohibited.
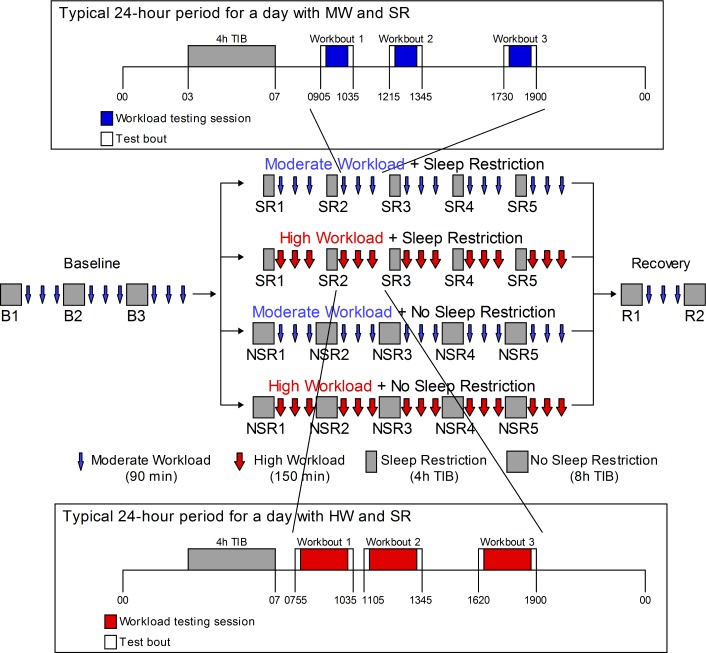
Figure 1 shows the experimental design for this protocol. In the laboratory phase, subjects received three baseline nights (B1-3) of 8 h TIB from 23:00-07:00. Following testing sessions after B3, subjects were randomized to either the MW or HW condition with (SR1-SR5) or without sleep restriction (NSR1-NSR5) for 5 nights (4 h TIB from 03:00-07:00 versus 8 h TIB from 23:00-07:00). The final 2 nights of the protocol were recovery nights (R1 and R2; 8 h TIB from 23:00-07:00).
Figure 1.
Experimental design for the laboratory study. Subjects received 3 baseline nights (B1-3) of 8 h time in bed (TIB) from 23:00-07:00. Following B3, subjects were randomized to either the moderate (MW) or high workload (HW) condition with sleep restriction (SR1-SR5) or without sleep restriction (NSR1-NSR5) for 5 nights (4 h TIB from 03:00-07:00 versus 8 h TIB from 23:00-07:00). The final 2 nights were recovery nights (R1 and R2; 8 h TIB from 23:00-07:00). Polysomnography was recorded on baseline night 3 (B3), experimental nights 1 (SR1, NSR1), 4 (SR4, NSR4), and 5 (SR5, NSR5), and R1. Subjects received three workload testing sessions per day of 120 min (HW) or 60 min (MW) of continuous work on a range of visually based cognitive tasks. They also completed a 15-min test bout consisting of the Psychomotor Vigilance Test, Karolinska Sleepiness Scale, and visual analog scale measures before and after workload tasks (30 min total). The MW testing sessions began at 09:05, 12:15, and 17:30 and lasted 90 min. The HW testing sessions began at 07:55, 11:05, and 16:20 and lasted 150 min (with a 10-min break). During baseline and recovery, subjects received MW testing sessions. Insets: 24-h day depicting workload sessions in the MW + SR condition (top) and in the HW + SR condition (bottom).
Workload Test Bouts and Neurobehavioral Measures
Subjects underwent three workload testing sessions during each protocol day (Figure 1). The MW testing sessions began at 09:05, 12:15, and 17:30 and lasted 90 min. The HW testing sessions began at 07:55, 11:05, and 16:20 and lasted 150 min (with a 10-min break). These times were selected to ensure the 90-min and 150-min workload bouts ended at the same times (i.e., 10:35, 13:45, and 19:00). The computerized cognitive workload test battery contained the following tasks: a serial addition and subtraction task44; a digit symbol substitution task45; a forward and backward digit span task45; a stroop task46; a go-no-go task47; a threat detection task48; a Windows spaceflight cognitive assessment task49; and an AusEd driving simulator task.50,51 Workload was manipulated via the amount of time engaged in cognitive test bouts, such that the duration of these tasks was doubled in the HW (120 min) versus the MW (60 min) conditions. In addition to these workload tasks, subjects performed a 15-min test bout before and after each workload testing session, for a total of 30 min. Thus, the posttest assessments of workload occurred at the same times of day across conditions. This test bout included the following assessments for evaluating the waking neurobehavioral effects of the four experimental conditions: a 10-min Psychomotor Vigilance Test (PVT),52,53 the Karolinska Sleepiness Scale (KSS),54 and a visual analog scale (VAS) of fatigue anchored by the concepts “fresh as a daisy” and “tired to death.”55 Two standardized executive function tests, the Hayling test56 and the Controlled Oral Word Association Test (COWAT),57 were also administered at NSR5/SR5.
In addition to the cognitive and subjective outcomes, a modified Maintenance of Wakefulness Test (MWT)6—a physiological measure of the ability to resist sleep—was administered at B3, NSR1/SR1, NSR4/SR4, and NSR5/SR5 (a single trial was conducted between 14:30 and 16:00) using a standard recording montage. Before each trial, the lights were dimmed to less than 10 lux and subjects were instructed to “keep your eyes open and try not to fall asleep.” Each trial was terminated at the first microsleep (10 sec of theta activity) determined by the C3-A2 derivation or at 30 min if sleep onset did not occur.6 MWT scores represented either the time (min) to microsleep initiation or 30 min (if no microsleep occurred).
PSG Measures
PSG recordings (EEG derivations Fz, C3, O2; Mastoids A1, A2; electrooculography [EOG] left outer canthus [LOC], right outer canthus [ROC]; submental electromyography [EMG]) were collected using digital ambulatory physiological recorders (Siesta 802.11 Ambulatory PSG System; Compumedics Limited, Abbotsford, Victoria, Australia) on B3, SR1/NSR1, SR4/ NSR4, SR5/NSR5, and R1. All sleep stages were scored visually in continuous 30-sec epochs according to Rechtschaffen and Kales58 by a trained scorer blind to experimental condition using commercial software (ProFusion PSG 3; Compumedics Limited). The EEGs and EOGs were referenced with A1 or A2 (Fz-A1, C3-A2, O2-A1, LOC-A2, and ROC-A1). A submental EMG was analyzed bipolarly. The sampling rate was 256 Hz. For sleep scoring, high-pass filters were set at 0.3 Hz for EEGs and EOGs and 10 Hz for EMG. Low-pass filters were set at 30 Hz for EEG and EOG and 100 Hz for EMG. SOL was defined as three consecutive 30-sec epochs of any sleep stage.
EEG Power Spectral Analysis
EEGs were subjected to power spectral analysis by applying a Fast Fourier transform. For spectral analyses, high-pass filters were set at 0.3 Hz and low-pass filters were set at 30 Hz. All PSG signals were analyzed by Vitascore software v1.50 (TEMEC Instruments, Kerkrade, The Netherlands). All signals, including EEG, EOG, and EMG, were visually inspected and fast- or slow-frequency artifacts such as body movement or excessive sweating were annotated as artifacts. Epochs that contained artifacts by visual inspection and by an automated artifact detection algorithm incorporated in Vitascore software v1.50 were not included. EEGs in Fz-A1, C3-A2 and O2-A1 were analyzed. If A1 or A2 contained severe artifacts, Fz-A2, C3-A1, or O2-A2 was used in order to maximize the number of channels for analysis. However, the same deviations were used for each subject across nights. Power spectra were computed per 4 sec by applying a Fast Fourier transform routine implemented in Vitascore. A squared cosine function was used as the tapering window and a 1-sec overlap between consecutive 4-sec epochs was applied. Power spectra between 0.5 Hz and 31.75 Hz for 10 4-sec epochs with 1-sec overlap were assigned the sleep stage of the visually scored 30-sec epoch.59,60 In this manuscript, we report SWA (0.5-4.5 Hz), SWE (0.5-4.5 Hz), and theta activity (4.75-7.75 Hz) during NREM sleep (stages 2-4) because our aim for power spectral analyses was to investigate the sleep homeostatic response. SWE was calculated as the integrated power in the SWA band (0.5-4.5 Hz) totaled over all 4-sec epochs of NREM sleep (stages 2-4) using the entire sleep period (i.e., 23:00-07:00 for the baseline night; 03:00-07:00 for the SR nights; and 23:00-07:00 for the R night). Power in SWA and theta activity were calculated by dividing the total energy of NREM (stages 2-4) sleep in each band by the number of NREM 4-sec epochs using the first 4-h sleep period (i.e., 23:00-03:00 for the baseline night; 03:00-07:00 for the SR nights; 23:00-03:00 for the NSR nights; and 23:00-03:00 for the R night).
Data Analyses
To establish the effects of night (SR1/NSR1, SR4/NSR4, SR5/NSR5, and R1), sleep conditions (SR/NSR), and workload conditions (HW/MW) on PSG measures, we performed mixed linear models with PROC MIXED (SAS v9.3, SAS Institute, Cary, NC, USA), which accommodate missing data. B3 measures, age, and sex were used as covariates. Subject was modeled as a random effect. Night, sleep condition, workload condition, and their interactions were modeled as fixed effects. The Kenward-Rogers method was used to calculate degrees of freedom.61 A spatial power covariance structure was used for experimental nights as repeated measures, which accounts for distances among nights because of our discontinuous sampling of PSG. In order to investigate the specific effects of the experimental conditions on EEG channels, we performed mixed linear models with PROC MIXED for SWA, SWE, and theta band activity on each condition (HW + SR, MW + SR, HW + NSR, or MW + NSR). Age and sex were used as covariates. Subject was modeled as a random effect. Night (SR1/NSR1, SR4/NSR4, SR5/NSR5, and R1), derivations (Fz, C3, O2), and their interactions were modeled as fixed effects, using the Kenward-Rogers method to calculate degrees of freedom.61 A direct product unstructured covariance was used for night and channel as repeated measures.
After determining NREM-REM cycles according to standard criteria,62 we performed PROC MIXED analyses for SWE, SWA, and theta band activity on each NREM cycle of the R night in the HW + SR condition. Age and sex were used as covariates and subject was modeled as a random effect. Derivation (Fz, C3, O2) was modeled as a fixed effect, using the Kenward-Rogers method to calculate degrees of freedom.61 An unstructured covariance matrix was used for derivations as repeated measures. Post hoc comparisons were made using pairwise comparisons with Tukey-Kramer corrections for multiple comparisons.
Mixed-model analyses of variance (ANOVAs), with study day as the within-subjects (repeated measures) factor, and HW/MW and SR/NSR as between-group factors, were used to analyze PVT, KSS, and VAS outcome data. The three daily postworkbout scores for PVT, KSS, and VAS fatigue for NSR1/SR1, NSR2/ SR2, NSR3/SR3, NSR4/SR4, NSR5/SR5, and R1 were included in the model to test the combined effect of workload and sleep restriction across experiment days. B3, test bout time of day, age, and sex were included as covariates. MWT SOL scores were analyzed similarly, except that time of day was not included in the model because the MWT was only administered once per day. One-way ANOVA was used to analyze executive functioning measures at NSR5/SR5. Post hoc comparisons were made using pairwise comparisons with Tukey-Kramer corrections for multiple comparisons. Effect sizes were calculated using Cohen's d (small, d = 0.2, medium, d = 0.5, large, d = 0.8).63
RESULTS
PSG Measures
PSG data are shown in Table 1 for each experimental night for the four conditions. All PSG measures except for sleep efficiency showed a significant interaction between sleep condition and protocol night (Table 2). SOL and wake after sleep onset (WASO) showed significant main effects of workload (Table 2). HW produced longer SOL than MW (Figure 2A; d = 0.63). After controlling for SR1/NSR1 night, baseline night 3, age, and sex, a significant effect of workload remained for SOL (three-way interaction among sleep condition, workload condition, and night; F2, 95.3 = 3.29, P = 0.04). SOL on the fifth night in the HW + NSR condition was longer than in the MW + NSR condition (Figure 2C, right graph; t 104.1 = 2.79, P = 0.007, d = 1.32, post hoc analyses). By contrast, there were no effects of workload on SOL in the SR conditions (all P > 0.05). HW produced less WASO than MW (Figure 2B; d = 0.64).
Table 1.
Polysomnographic measures for experimental nights 1 (SR1/NSR1), 4 (SR4/NSR4), 5 (SR5/NSR5) and recovery night 1 (R1).
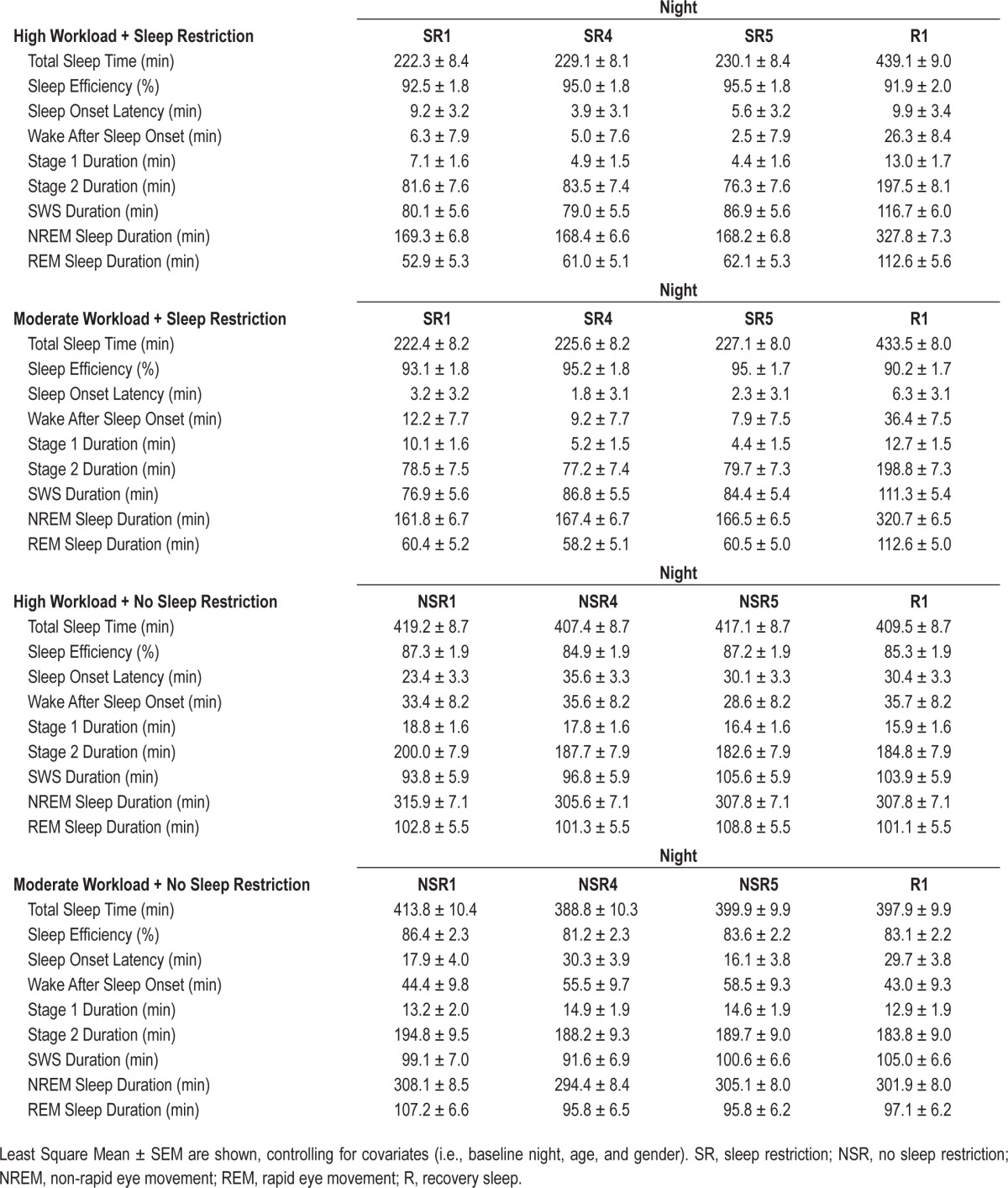
Table 2.
Effects of conditions and interactions for polysomnographic measures.
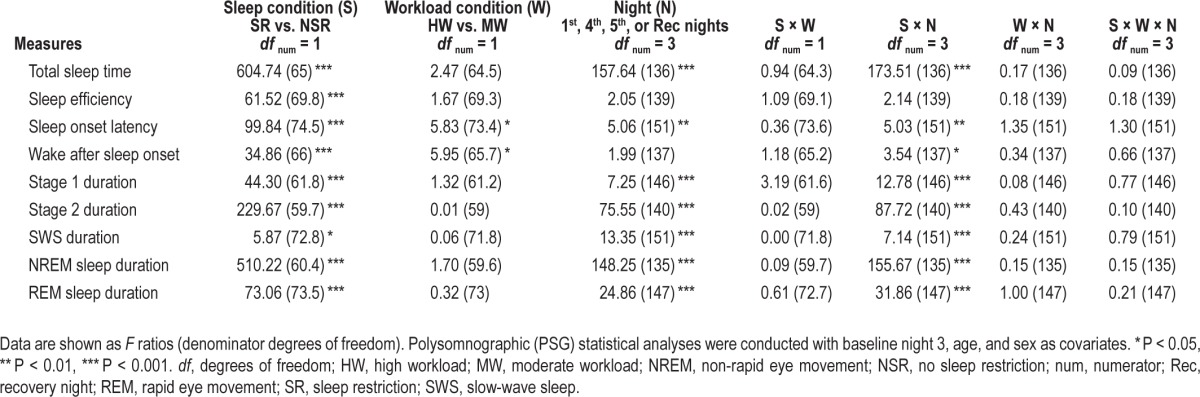
Figure 2.
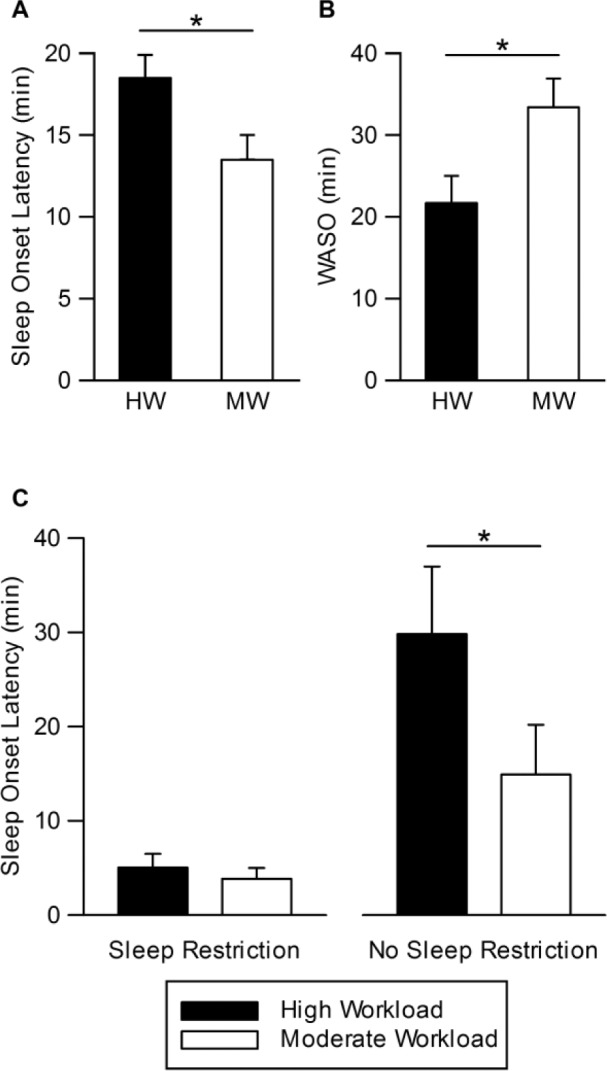
Effect of cognitive workload on sleep onset latency and wake after sleep onset. (A) Main effect of workload on sleep onset latency (SOL) and (B) wake after sleep onset (WASO), whereby HW produced longer SOL (d = 0.63) but shorter WASO (d = 0.64) than MW. Least Square Mean ± standard error of the mean (SEM), controlling for covariates (baseline night 3, age, and sex). (C) Mean (± SEM) sleep onset latency on the fifth protocol night. SOL on the fifth night in the high workload + no sleep restriction (HW + NSR) condition was longer than that in the moderate workload + no sleep restriction (MW + NSR) condition (right graph), indicating higher cognitive workload delayed sleep onset when there was no additional sleep pressure from sleep restriction. * P < 0.05. HW, high workload; MW, moderate workload.
SWA, SWE, and Theta Band Activity
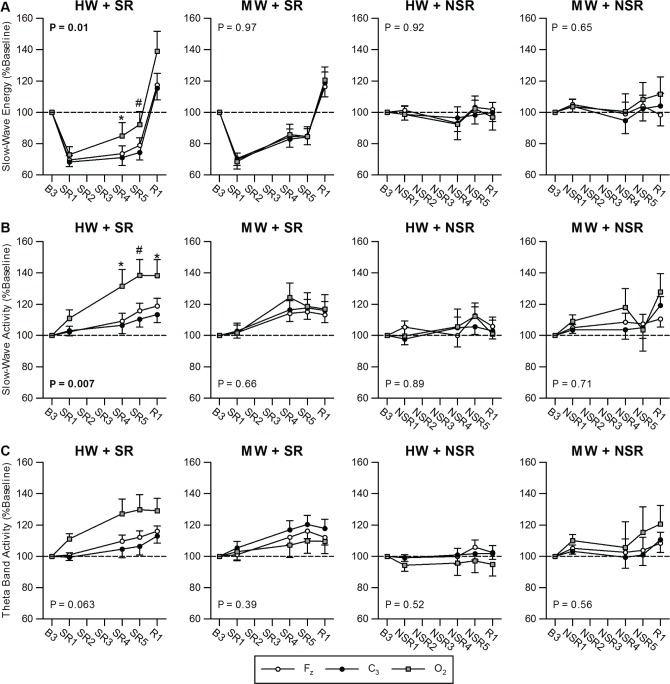
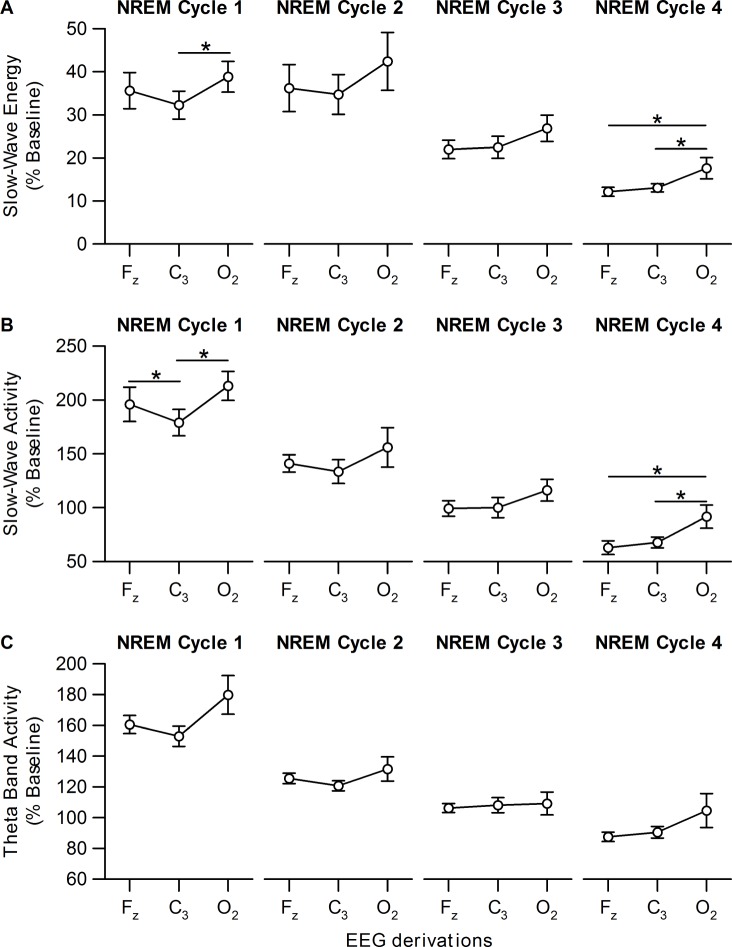
Table 3 summarizes the channel effects for EEG measures. Figure 3 shows SWE, SWA, and theta band activity in the four conditions. Only the HW + SR condition showed a main effect of channel on SWA and SWE: SWA and SWE for O2 were higher than for C3 (SWE: t 24.1 = 2.63, P = 0.01; SWA: t 25.1 = 3.11, P = 0.01; post hoc analyses). After controlling for SR1 night, age, and sex, a significant main effect of channel remained for SWA in the HW + SR condition (F 2, 19.6 = 4.45, P = 0.03). We further examined the time course of higher O2 SWE and SWA in the HW + SR condition during the R night. SWE was significantly higher in the O2 than C3 channel in the first NREM sleep cycle (Figure 4A). Similarly, SWA in the Fz and O2 channels was significantly higher than in the C3 channel in the first NREM sleep cycle (Figure 4B). Moreover, SWE and SWA were significantly higher in the O2 channel than in the Fz and C3 channels in NREM cycle 4, suggesting the local (occipital) increase of sleep homeostasis persisted over the entire R night sleep period (Figure 4).
Table 3.
Channel effects for slow-wave energy, slow-wave activity, and theta band activity.
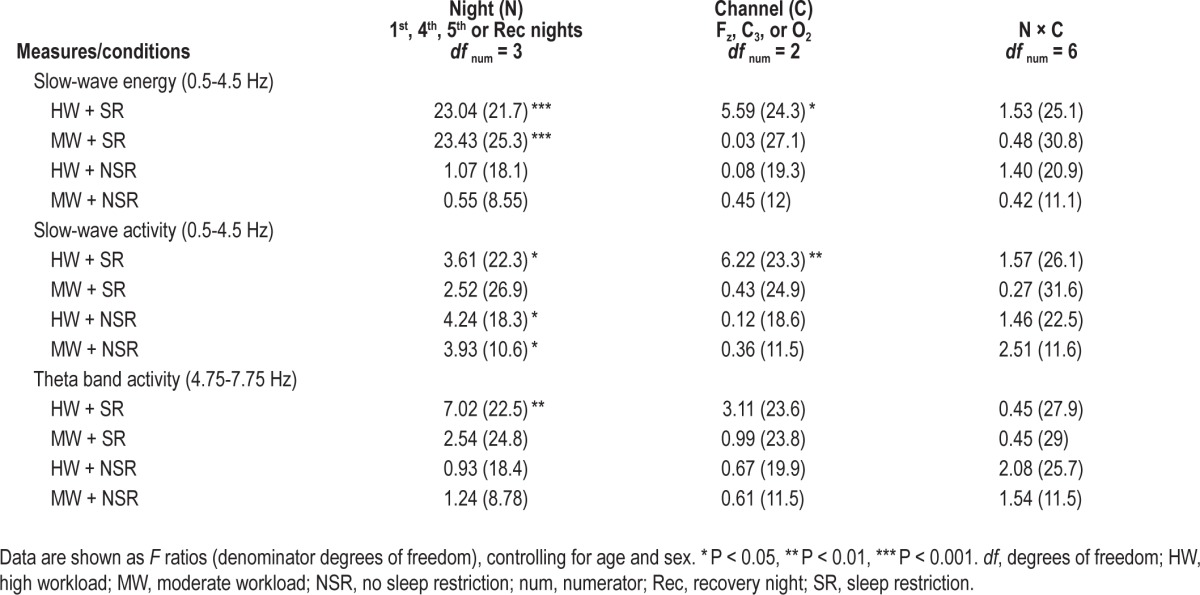
Figure 3.
Slow-wave energy (SWE, 0.5-4.5 Hz), slow-wave activity (SWA, 0.5-4.5 Hz), and theta band electroencephalographic (EEG) activity (4.75-7.75 Hz) in the Fz, O2, and C3 EEG channels for the four conditions. (A) SWE, (B) SWA, and (C) theta band activity responses on experimental night 1 (SR1/NSR1), experimental night 4 (SR4/NSR4), experimental night 5 (SR5/NSR5) and recovery night (R1) in the high workload + sleep restriction condition (HW + SR), moderate workload + sleep restriction condition (MW + SR), high workload + no sleep restriction condition (HW + NSR), and moderate workload + no sleep restriction condition (MW + NSR). Only the HW + SR condition yielded a main effect of channel for SWE and SWA, indicating locally increased sleep homeostatic responses to visual cognitive tasks in the occipital region in this condition. After controlling for SR1, age, and sex, SWA in the HW + SR condition showed a significant main effect of channel (F 2, 19.6 = 4.45, P = 0.025). Theta band activity did not show significant effects of channel. No significant interactions between channel and night were found (see also Table 3). P values listed in the figure are for a main effect of channel. * P < 0.05, # P < 0.01. NSR, no sleep restriction; R, recovery; SR, sleep restriction.
Figure 4.
Slow-wave energy (SWE), slow-wave activity (SWA), and theta band activity changes in the Fz, O2, and C3 electroencephalographic (EEG) derivations in each non-rapid eye movement (NREM) cycle during recovery night 1 for the high workload + sleep restriction condition. (A) SWE, (B) SWA, and (C) theta band activity responses on recovery night (R1) in the high workload + sleep restriction condition (HW + SR). The SWE, SWA, and theta band activity for each NREM cycle was expressed as a percentage of the mean SWE, SWA, and theta band activity, respectively, in all NREM sleep periods during the 8-h baseline night. SWE in the occipital (O2) region was significantly higher than in the central (C3) region in the first NREM sleep cycle. Similarly, SWA in the frontal (Fz) and O2 regions was significantly higher than in the C3 region in the first NREM sleep cycle. SWE and SWA were significantly higher in the O2 derivation than in the Fz and C3 regions in NREM cycle 4, suggesting the local (O2) increase of sleep homeostasis persisted over the entire recovery night sleep period. Least squares mean (± standard error of the mean), controlling for covariates (age and sex). * P < 0.05. NREM, non rapid-eye movement.
Subjective Sleepiness and Fatigue
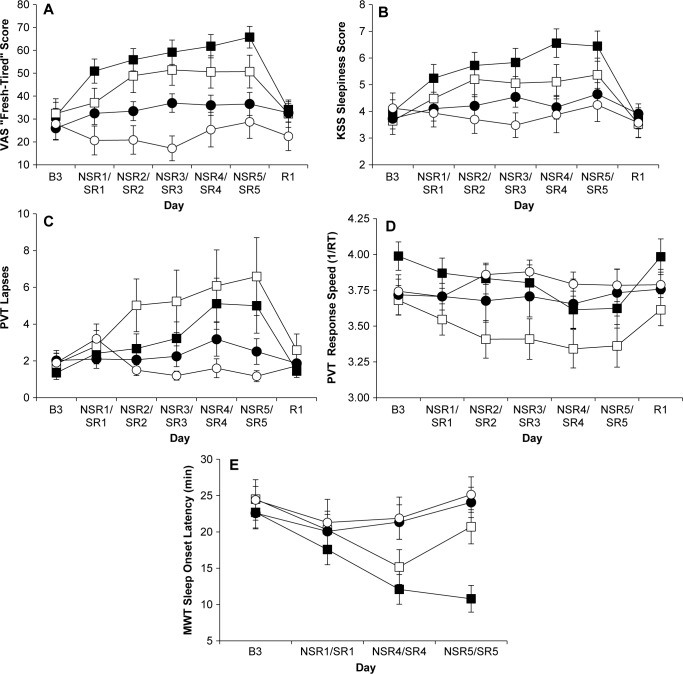
VAS subjective fatigue (F 1, 66 = 14.56, P = 0.0003) and KSS sleepiness scores (F 1, 59 = 5.65, P = 0.021) showed a significant main effect of workload: HW elicited greater feelings of both fatigue (mean HW = 45.58; MW = 33.48) and sleepiness (mean HW = 4.93; MW = 4.27) than MW (Figure 5A and 5B). VAS fatigue (F5, 120 = 9.61, P < 0.0001) and KSS sleepiness (F5, 121 = 8.85, P < 0.0001) also showed significant SR/NSR × Day interactions. Furthermore, VAS fatigue (F5, 120 = 29.07, P < 0.0001) and KSS sleepiness scores (F5, 121 = 28.59, P < 0.0001) showed significant SR × Day interactions, but no significant NSR × Day interactions (VAS fatigue: F5, 120 = 1.53, P = 0.19; KSS sleepiness: F5, 122 = 1.61, P = 0.16). Thus, VAS fatigue and KSS sleepiness ratings increased across the five experimental days in the SR but not the NSR conditions.
Figure 5.
Neurobehavioral data for the four experimental conditions. Mean (± standard error of the mean) postbout (A) “fresh-tired” visual analog scale (VAS) fatigue scale scores; (B) Karolinska Sleepiness Scale (KSS) scores; (C) Psychomotor Vigilance Test (PVT) lapses; (D) PVT response speed (1/RT); and (E) Maintenance of Wakefulness Test (MWT) sleep onset latencies averaged across a day for the HW + SR (filled squares), MW + SR (open squares), HW + NSR (filled circles) and MW + NSR (open circles) groups. B3, baseline night 3; NSR/SR, no sleep restriction or sleep restriction days; R1, recovery day 1. See text for a description of the statistical results from these graphs.
Cognitive Performance and Executive Functioning
PVT lapses and response speed showed significant SR/NSR × Day interactions: there were differential cognitive performance deficits across experiment days as demonstrated by increases in PVT performance lapses (F 5, 111 = 4.55, P = 0.0008) and decreases in PVT response speed (F 5, 117 = 7.45, P < 0.0001; Figures 5C and 5D). Furthermore, PVT lapses (F5, 111 = 9.89, P < 0.0001) and response speed (F5, 117 = 18.72, P < 0.0001) showed significant SR × Day interactions, but no significant NSR × Day interactions (lapses (F5, 111 = 1.02, P = 0.41; response speed (F5, 117 = 1.01, P = 0.42). Thus, PVT performance deteriorated across the five experimental days in the SR conditions but not the NSR conditions. By contrast, neither PVT lapses nor response speed showed significant main effects of workload. In addition, no significant group differences were found for the Hayling (F3, 61 = 1.436, P = 0.24) or the COWAT (F3, 61 = 0.746, P = 0.53) executive functioning tests conducted at NSR5/SR5.
Physiologic Alertness
The MWT showed a significant SR/NSR × Day interaction (F2, 109 = 6.61, P = 0.019; Figure 5E) whereby SR produced a decreased ability to resist sleep (mean SR SOL at SR5 = 16.17 min) compared with NSR (mean NSR SOL at SR5 = 25.58 min). MWT SOL did not show a significant effect of workload.
DISCUSSION
In this experiment, high workload in the absence of sleep loss, increased subjective fatigue, and sleepiness and initially activated the wake-promoting system by delaying sleep onset and subsequently activated the sleep-promoting system by increasing global homeostatic responses as evidenced by less time awake after sleep onset. HW increased local, presumably use-dependent, homeostatic responses as evidenced by increased SWA in the occipital region, in response to visual cognitive tasks during days with and without SR. Thus, our results indicate that two waking neurobehavioral factors—SR (i.e., duration of wakefulness) and cognitive workload (i.e., duration of work)—affect sleep physiology, behavioral alertness, and subjective reactions as both separate and interacting forces. Their additive interaction was especially evident in the increase in SWE over the occipital cortex during sleep, following HW combined with SR, suggesting that elevated workload increased the intensity of sleep homeostasis.
The occipital derivation showed higher SWA and SWE during SR and R in the HW + SR condition compared to the central derivation, consistent with an experiment in which we found greater increases in O2 SWA during R sleep after high workload during acute total sleep deprivation.39 The finding is also consistent with previous reports demonstrating use-dependent local increases of SWA in specific areas related to an experimental task or stimulation site.24–26,40 All of the tasks in our workload sessions were presented primarily using visual modalities; thus, the occipital cortex was actively engaged in performing throughout the test batteries. Therefore, a greater increase in SWA and SWE in the O2 derivation may be caused by local use-dependent sleep homeostatic processes induced by waking cognitive activity (especially visual neural processing). However, because we did not evaluate the effects of other types of neurobehavioral tests (e.g., auditory or tactile tasks), we cannot be certain the elevated sleep homeostatic response we observed over the occipital cortex was unique to visual performance. However, humans process visual input during most of the time they are awake (assuming they have intact sight), which may prioritize sleep homeostatic responses in the occipital lobe.
The current study demonstrated that high cognitive workload delayed SOL, specifically on the fifth night in HW + NSR condition, when there was no additional sleep pressure from SR. This result is in line with previous studies that found SOL increased after performing cognitive tasks.19,22,23 For example, Higuchi and colleagues found that playing a computer game before going to bed (23:00-01:45) delayed SOL and increased heart rate, suggesting that increased SOL was caused by increased central and autonomic nervous system activation.22 Wuyts and colleagues also reported that 30 min of various cognitive tasks from 21:25 to 21:55 delayed SOL without negatively affecting emotions before bedtime.23 Thus, one possible reason for the delayed sleep onset observed in our study is that HW caused sustained activation of the central and/or autonomic nervous systems.
In the current study, HW reduced WASO compared with MW. This finding is consistent with a study by de Bruin and colleagues21 demonstrating that subjects had less WASO following HW (8 h of cognitive tasks including sustained attention, memory, logical thinking, decision making, and calculating tasks) than after low cognitive workload (8 h of video watching). Notably, WASO relates to, and may be an index of, sleep pressure: WASO increases as time since sleep progresses, increases with age,64,65 and increases following use of the stimulant caffeine.66 In contrast, hypnotic medication67 and sleep restriction,27,28,31,33,34 both which affect sleep homeostasis, decrease WASO. Thus, we conclude that sleep homeostasis increased as evidenced by WASO and SWA/SWE in the HW + SR condition. Furthermore, it is likely that subjects in the HW conditions had higher sleep pressure than those in the MW conditions, which might also explain higher ratings of fatigue and sleepiness in those conditions. A recent study in mice also found a similar dissociation of arousal and homeostatic sleep need: emotional, behavioral, and physiological arousal evoked by changing of the home cage induced longer SOLs and increased NREM delta EEG power following sleep deprivation compared to gentle handling.68
The flip-flop switch model suggests that sleep- and wake-promoting neurons inhibit each other and that orexin/hypocretin neurons help stabilize wakefulness and sleep.69 We speculate that HW combined with SR induced elevated activity in both the wake-promoting system and the sleep-promoting system. This could result in an initial activation of the wake-promoting system, manifesting as delayed sleep onset, followed by a subsequent activation of the sleep-promoting system, manifesting as decreased WASO and increased SWA/SWE to reflect increases in both global and local sleep homeostasis, respectively.
Our experiment had several limitations. As noted previously, subjects performed cognitive tasks primarily using visual inputs. Cognitive tasks using another modality such as auditory stimulation might result in greater local increases in different areas such as those over the auditory cortex. Our workload tasks ended at 19:00, several hours before bedtime. There is a possibility that conducting cognitive tasks closer to bedtime may result in longer or shorter SOLs than we observed or larger differences in waking neurobehavioral outcomes. In addition, research is needed to investigate the effects on sleep physiology and neurobehavioral performance of HW at different times across a 24-h cycle. Finally, our workload manipulation was defined by doubling the duration of the same cognitive tasks, to avoid differential task effects on sleep responses. Therefore, our results may not generalize to the effects of other aspects of workload such as task difficulty or variations in the pace of work on sleep physiology and fatigue.
CONCLUSIONS
This experiment revealed a combination of high cognitive (visual) workload and sleep restriction of 4 h for 5 nights resulted in higher global and local (occipital) increase of sleep homeostasis as evidenced by both decreased WASO and increased SWA/SWE. The results suggest that the interaction of high workloads and sleep restriction also produced greater waking sleepiness and fatigue. They add to a much-needed understanding of the role daily sleep time has in recovery of cognitive functions under conditions that simulate work-rest schedules.
DISCLOSURE STATEMENT
This was not an industry-supported study. This work was supported by the National Space Biomedical Research Institute through NASA NCC 9-58; by the CTRC UL1 TR000003; by the Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad and by NIH T32 HL07713. Drs. Abe and Braun have no financial conflicts of interest. Dr. Goel is an Associate Editor of SLEEP and has received compensation for serving as a consultant on NASA and NSBRI grants, for serving as a NIH and DoD study section member and National Sleep Foundation expert panel member, and for lectures delivered at the University of Pittsburgh Medical Center, Drexel University College of Medicine, the World Federation of Sleep Research & Sleep Medicine Societies meeting, and the Society for Light Treatment and Biological Rhythms meeting. Dr. Dinges is compensated by the Associated Professional Sleep Societies, LLC, for serving as Editor in Chief of SLEEP and has received compensation for serving on a scientific advisory council for Mars, Inc. Drs. Goel and Dinges recuse themselves from all decisions related to SLEEP manuscripts on which they have a conflict of interest. Work was performed at the Division of Sleep and Chronobiology, Unit for Experimental Psychiatry, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA.
ACKNOWLEDGMENTS
Drs. Goel and Abe are co-first authors and contributed equally to this work. The authors thank the faculty and staff of the Unit of Experimental Psychiatry for their contributions to this study.
Footnotes
A commentary on this article appears in this issue on page 1727.
REFERENCES
- 1.Mitler MM, Carskadon MA, Czeisler CA, Dement WC, Dinges DF, Graeber RC. Catastrophes, sleep, and public-policy: consensus report. Sleep. 1988;11:100–9. doi: 10.1093/sleep/11.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinges DF. An overview of sleepiness and accidents. J Sleep Res. 1995;4:4–14. doi: 10.1111/j.1365-2869.1995.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 3.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 4.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 5.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 6.Banks S, Van Dongen HP, Maislin G, Dinges DF. Neurobehavioral dynamics following chronic sleep restriction: dose-response effects of one night for recovery. Sleep. 2010;33:1013–26. doi: 10.1093/sleep/33.8.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim J, Wu WC, Wang J, Detre JA, Dinges DF, Rao H. Imaging brain fatigue from sustained mental workload: An ASL perfusion study of the time-on-task effect. Neuroimage. 2010;49:3426–35. doi: 10.1016/j.neuroimage.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macdonald W, Bendak S. Effects of workload level and 8-versus 12-h workday duration on test battery performance. Int J Ind Ergon. 2000;26:399–416. [Google Scholar]
- 9.Åkerstedt T, Fredlund P, Gillberg M, Jansson B. Work load and work hours in relation to disturbed sleep and fatigue in a large representative sample. J Psychosomat Res. 2002;53:585–8. doi: 10.1016/s0022-3999(02)00447-6. [DOI] [PubMed] [Google Scholar]
- 10.Landrigan CP, Rothschild JM, Cronin JW, et al. Effect of reducing interns' work hours on serious medical errors in intensive care units. N Engl J Med. 2004;351:1838–48. doi: 10.1056/NEJMoa041406. [DOI] [PubMed] [Google Scholar]
- 11.Rogers AE, Hwang WT, Scott LD, Aiken LH, Dinges DF. The working hours of hospital staff nurses and patient safety. Health Aff. 2004;23:202–12. doi: 10.1377/hlthaff.23.4.202. [DOI] [PubMed] [Google Scholar]
- 12.Barger LK, Cade BE, Ayas NT, et al. Extended work shifts and the risk of motor vehicle crashes among interns. N Engl J Med. 2005;352:125–34. doi: 10.1056/NEJMoa041401. [DOI] [PubMed] [Google Scholar]
- 13.Philibert I. Sleep loss and performance in residents and nonphysicians: a meta-analytic examination. Sleep. 2005;28:1392–402. doi: 10.1093/sleep/28.11.1392. [DOI] [PubMed] [Google Scholar]
- 14.Abe T, Komada Y, Nishida Y, Hayashida K, Inoue Y. Short sleep duration and long spells of driving are associated with the occurrence of Japanese drivers' rear-end collisions and single-car accidents. J Sleep Res. 2010;19:310–6. doi: 10.1111/j.1365-2869.2009.00806.x. [DOI] [PubMed] [Google Scholar]
- 15.Dorrian J, Baulk SD, Dawson D. Work hours, workload, sleep and fatigue in Australian Rail Industry employees. Appl Ergon. 2011;42:202–9. doi: 10.1016/j.apergo.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Åkerstedt T, Knutsson A, Westerholm P, Theorell T, Alfredsson L, Kecklund G. Sleep disturbances, work stress and work hours - A cross-sectional study. J Psychosomat Res. 2002;53:741–8. doi: 10.1016/s0022-3999(02)00333-1. [DOI] [PubMed] [Google Scholar]
- 17.Ribet C, Derriennic F. Age, working conditions, and sleep disorders: a longitudinal analysis in the French cohort ESTEV. Sleep. 1999;22:491–504. [PubMed] [Google Scholar]
- 18.Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557–68. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 19.Koulack D, Prevost F, Dekoninck J. Sleep, dreaming, and adaptation to a stressful intellectual activity. Sleep. 1985;8:244–53. doi: 10.1093/sleep/8.3.244. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi M, Arito H. Suppression of electroencephalogram-delta power density during non-rapid eye-moement sleep as a result of a prolonged cognitive task prior to sleep onset. Eur J Appl Physiol Occup Physiol. 1994;68:274–80. doi: 10.1007/BF00376777. [DOI] [PubMed] [Google Scholar]
- 21.de Bruin EA, Beersma DGM, Daan S. Sustained mental workload does not affect subsequent sleep intensity. J Sleep Res. 2002;11:113–21. doi: 10.1046/j.1365-2869.2002.00290.x. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi S, Motohashi Y, Liu Y, Maeda A. Effects of playing a computer game using a bright display on presleep physiological variables, sleep latency, slow wave sleep and REM sleep. J Sleep Res. 2005;14:267–73. doi: 10.1111/j.1365-2869.2005.00463.x. [DOI] [PubMed] [Google Scholar]
- 23.Wuyts J, De Valck E, Vandekerckhove M, et al. The influence of pre-sleep cognitive arousal on sleep onset processes. Int J Psychophysiol. 2012;83:8–15. doi: 10.1016/j.ijpsycho.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Kattler H, Dijk DJ, Borbély AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–64. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 25.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 26.Huber R, Esser SK, Ferrarelli F, Massimini M, Peterson MJ, Tononi G. TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS One. 2007;2:e276. doi: 10.1371/journal.pone.0000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunner DP, Dijk DJ, Tobler I, Borbély AA. Effect of partial sleep-deprivation on sleep stages and EEG power spectra: evidence for non-REM and REM-sleep homeostasis. Electroencephalogr Clin Neurophysiol. 1990;75:492–9. doi: 10.1016/0013-4694(90)90136-8. [DOI] [PubMed] [Google Scholar]
- 28.Brunner DP, Dijk DJ, Borbély AA. Repeated partial sleep deprivation progressively changes in EEG during sleep and wakefulness. Sleep. 1993;16:100–13. doi: 10.1093/sleep/16.2.100. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y, Laposky AD, Bergmann BM, Turek FW. Repeated sleep restriction in rats leads to homeostatic and allostatic responses during recovery sleep. Proc Natl Acad Sci U S A. 2007;104:10697–702. doi: 10.1073/pnas.0610351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Åkerstedt T, Kecklund G, Ingre M, Lekander M, Axelsson J. Sleep homeostasis during repeated sleep restriction and recovery: support from EEG dynamics. Sleep. 2009;32:217–22. doi: 10.1093/sleep/32.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goel N, Banks S, Mignot E, Dinges DF. PER3 polymorphism predicts cumulative sleep homeostatic but not neurobehavioral changes to chronic partial sleep deprivation. PLoS One. 2009;4:e5874. doi: 10.1371/journal.pone.0005874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leemburg S, Vyazovskiy VV, Olcese U, Bassetti CL, Tononi G, Cirelli C. Sleep homeostasis in the rat is preserved during chronic sleep restriction. Proc Natl Acad Sci U S A. 2010;107:15939–44. doi: 10.1073/pnas.1002570107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goel N, Banks S, Mignot E, Dinges DF. DQB1*0602 predicts interindividual differences in physiologic sleep, sleepiness, and fatigue. Neurology. 2010;75:1509–19. doi: 10.1212/WNL.0b013e3181f9615d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goel N, Banks S, Lin L, Mignot E, Dinges DF. Catechol-O-methyltransferase Val158Met polymorphism associates with individual differences in sleep physiologic responses to chronic sleep loss. PLoS One. 2011;6:e29283. doi: 10.1371/journal.pone.0029283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cajochen C, Foy R, Dijk DJ. Frontal predominance of a relative increase in sleep delta and theta EEG activity after sleep loss in humans. Sleep Res Online. 1999;2:65–9. [PubMed] [Google Scholar]
- 37.Finelli LA, Borbély AA, Achermann P. Functional topography of the human nonREM sleep electroencephalogram. Eur J Neurosci. 2001;13:2282–90. doi: 10.1046/j.0953-816x.2001.01597.x. [DOI] [PubMed] [Google Scholar]
- 38.Münch M, Knoblauch V, Blatter K, et al. The frontal predominance in human EEG delta activity after sleep loss decreases with age. Eur J Neurosci. 2004;20:1402–10. doi: 10.1111/j.1460-9568.2004.03580.x. [DOI] [PubMed] [Google Scholar]
- 39.Caruso HM, Stakofsky A, Tucker A, Dinges DF, Van Dongen H. Effect of cognitive workload on delta power in the NREM EEG of recovery sleep following acute sleep deprivation. Sleep. 2006;29:A135–6. Abstract Suppl. [Google Scholar]
- 40.Hung CS, Sarasso S, Ferrarelli F, et al. Local experience-dependent changes in the wake EEG after prolonged wakefulness. Sleep. 2013;36:59–72. doi: 10.5665/sleep.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stakofsky AB, Levin AL, Vitellaro KM, Dinges DF, Van Dongen H. Effect of cognitive workload on neurobehavioral deficits during total sleep deprivation. Sleep. 2005;28:A128–9. Abstract Suppl. [Google Scholar]
- 42.Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74:728–38. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- 43.Douglass AB, Bornstein R, Nino-Murcia G, et al. The Sleep Disorders Questionnaire. I: Creation and multivariate structure of SDQ. Sleep. 1994;17:160–7. doi: 10.1093/sleep/17.2.160. [DOI] [PubMed] [Google Scholar]
- 44.Thorne DR, Genser SG, Sing HC, Hegge FW. The Walter Reed performance assessment battery. Neurobehav Toxicol Teratol. 1985;7:415–8. [PubMed] [Google Scholar]
- 45.Wechsler Adult Intelligence Scale 3 Technical Manual. San Antonio, TX: Harcourt Brace and Company; 1997. [Google Scholar]
- 46.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;28:643–62. [Google Scholar]
- 47.Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96:8301–6. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basner M, Rubinstein J, Fomberstein KM, et al. Effects of night work, sleep loss and time on task on simulated threat detection performance. Sleep. 2008;31:1251–9. [PMC free article] [PubMed] [Google Scholar]
- 49.Kane RL, Short P, Sipes W, Flynn CF. Development and validation of the spaceflight cognitive assessment tool for windows (WinSCAT) Aviat Space Environ Med. 2005;76:B183–91. [PubMed] [Google Scholar]
- 50.Banks S, Catcheside P, Lack LC, Grunstein RR, McEvoy RD. The Maintenance of Wakefulness Test and driving simulator performance. Sleep. 2005;28:1381–5. doi: 10.1093/sleep/28.11.1381. [DOI] [PubMed] [Google Scholar]
- 51.Desai AV, Wilsmore B, Bartlett DJ, et al. The utility of the AusEd driving simulator in the clinical assessment of driver fatigue. Behav Res Methods. 2007;39:673–81. doi: 10.3758/bf03193039. [DOI] [PubMed] [Google Scholar]
- 52.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 53.Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34:581–91. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 55.Monk TH. A visual analogue scale technique to measure global vigor and affect. Psychiatry Res. 1989;27:89–99. doi: 10.1016/0165-1781(89)90013-9. [DOI] [PubMed] [Google Scholar]
- 56.Burgess PW, Shallice T. The Hayling and Brixton tests. Bury St. Edmunds: Thames Valley Test Company Limited; 1997. [Google Scholar]
- 57.Benton AL, deS Hamsher K. Multilingual aphasia examination. Iowa City, IA: University of Iowa; 1976. [Google Scholar]
- 58.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington DC: Public Health Service, US Government Printing Office; 1968. [Google Scholar]
- 59.Van Reen E, Tarokh L, Rupp TL, Seifer R, Carskadon MA. Does timing of alcohol administration affect sleep? Sleep. 2011;34:195–205. doi: 10.1093/sleep/34.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walsh JK, Deacon S, Dijk DJ, Lundahl J. The selective extrasynaptic GABA(A) agonist, gaboxadol, improves traditional hypnotic efficacy measures and enhances slow wave activity in a model of transient insomnia. Sleep. 2007;30:593–602. doi: 10.1093/sleep/30.5.593. [DOI] [PubMed] [Google Scholar]
- 61.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–97. [PubMed] [Google Scholar]
- 62.Feinberg I, Floyd TC. Systematic trends across the night in human sleep cycles. Psychophysiology. 1979;16:283–91. doi: 10.1111/j.1469-8986.1979.tb02991.x. [DOI] [PubMed] [Google Scholar]
- 63.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 64.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 65.Dijk DJ, Groeger JA, Stanley N, Deacon S. Age-related reduction in daytime sleep propensity and nocturnal slow wave sleep. Sleep. 2010;33:211–23. doi: 10.1093/sleep/33.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karacan I, Thornby JI, Anch AM, Booth GH, Williams RL, Salis PJ. Dose-related sleep disturbances induced by coffee and caffeine. Clin Pharmacol Ther. 1976;20:682–9. doi: 10.1002/cpt1976206682. [DOI] [PubMed] [Google Scholar]
- 67.Paterson LM, Nutt DJ, Ivarsson M, Hutson PH, Wilson SJ. Effects on sleep stages and microarchitecture of caffeine and its combination with zolpidem or trazodone in healthy volunteers. J Psychopharmacol. 2009;23:487–94. doi: 10.1177/0269881109104852. [DOI] [PubMed] [Google Scholar]
- 68.Suzuki A, Sinton CM, Greene RW, Yanagisawa M. Behavioral and biochemical dissociation of arousal and homeostatic sleep need influenced by prior wakeful experience in mice. Proc Natl Acad Sci U S A. 2013;110:10288–93. doi: 10.1073/pnas.1308295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]