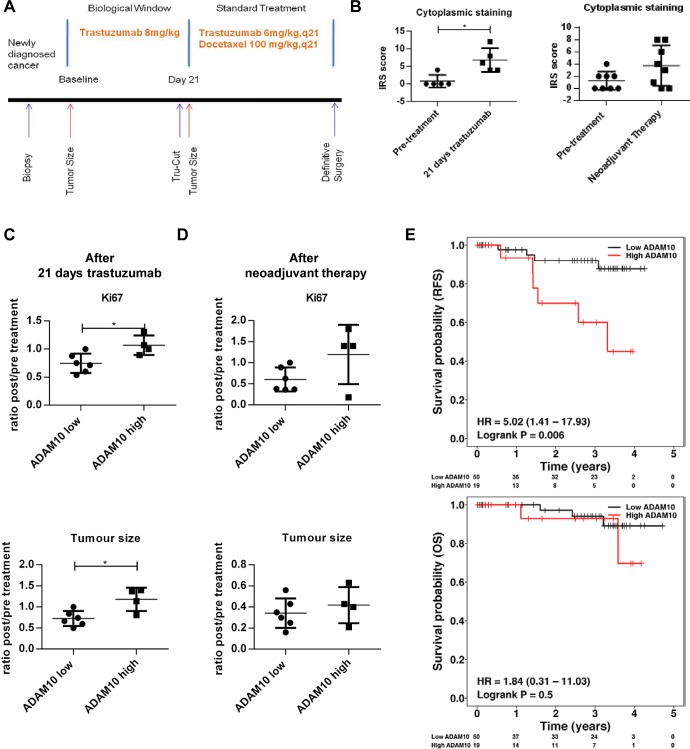
Figure 7. ADAM10 level is a predictive biomarker for trastuzumab response and is prognostic in a cohort of HER2 positive breast cancer patients.
(A) Schematic illustration of the window of opportunity study. HER2 positive breast cancer patients received a pre-treatment biopsy and underwent a 21 day trastuzumab (8mg/kg) monotherapy window study before a second biopsy was performed. Patients further received 4 cycles of neoadjuvant docetaxel chemotherapy 100 mg/m2 with 6mg/kg trastuzumab q21 prior to definitive surgery. (B) Paired tissue samples (pre- and post-treatment) from 5 patients who received trastuzumab monotherapy for 21 days (as described in A) and from 8 patients (of whom samples of pre- and post-neoadjuvant treatment were available) were stained for ADAM10 expression. (C) and (D) Basal ADAM10 expression levels (low or high) of a total of 10 patients (of whom basal biopsies and clinical data were available) were correlated with Ki67 staining and tumor size at day 21 and at definitive surgery (ratios between post trastuzumab or neoadjuvant treatment vs. pre-treatment). Bar graphs show means ± SD, the differences were assessed by t-Test and a p-value is shown (*p≤0.05). (E) Tissue microarrays (TMAs) consisting of tumor core samples from a well annotated HER2 positive breast cancer cohort were stained for basal ADAM10 expression by IHC. Relapse-free survival and overall survival of patients were plotted according to high or low ADAM10 IRS scores and differences between the groups were assessed using Log-rank test.