ABSTRACT
The histone-like nucleoid-structuring (H-NS) protein binds to horizontally acquired genes in the bacterium Salmonella enterica serovar Typhimurium, silencing their expression. We now report that overcoming the silencing effects of H-NS imposes a delay in the expression of genes activated by the transcriptional regulator PhoP. We determine that PhoP-activated genes ancestral to Salmonella are expressed before those acquired horizontally. This expression timing reflects the in vivo occupancy of the corresponding promoters by the PhoP protein. These results are surprising because some of these horizontally acquired genes reached higher mRNA levels than ancestral genes expressed earlier and were transcribed from promoters harboring PhoP-binding sites with higher in vitro affinity for the PhoP protein. Our findings challenge the often-made assumption that for genes coregulated by a given transcription factor, early genes are transcribed to higher mRNA levels than those transcribed at later times. Moreover, they provide a singular example of how gene ancestry can impact expression timing.
IMPORTANCE
We report that gene ancestry dictates the expression behavior of genes under the direct control of the Salmonella transcriptional regulator PhoP. That is, ancestral genes are transcribed before horizontally acquired genes. This reflects both the need to overcome silencing by the H-NS protein of the latter genes and the architecture of the corresponding promoters. Unexpectedly, transcription levels do not reflect transcription timing. Our results illustrate how a bacterium can exhibit an elaborate temporal expression behavior among genes coregulated by a transcription factor even though the products encoded by the target genes do not participate in a morphological or developmental pathway.
INTRODUCTION
The primary mechanism governing inducible gene expression operates at the level of transcription initiation, whereby a DNA binding protein recognizes specific sequences in promoters to activate or repress gene transcription (1, 2). For genes under the control of a transcriptional activator, the affinity of the transcription factor for its target sequences often dictates recruitment of RNA polymerase (RNAP) and the resulting transcriptional output (i.e., mRNA levels). However, this is not always the case because nucleoid-associated proteins can hinder access to the sequences recognized by a transcriptional activator (3, 4).
In the enteric bacteria Salmonella enterica serovar Typhimurium (5, 6) and Escherichia coli (7), the histone-like nucleoid-structuring (H-NS) protein (4) binds preferentially to AT-rich DNA sequences in their genomes, silencing expression of the corresponding genes. H-NS appears to impact preferentially horizontally acquired genes because they tend to have a higher AT content than ancestral genes (4, 8). Inactivation of the gene specifying H-NS derepresses expression of horizontally acquired genes (9). Therefore, the specific growth conditions and/or regulatory proteins that trigger expression of horizontally acquired genes function, at least in part, by overcoming silencing by H-NS. However, it is presently unknown whether and how overcoming silencing by H-NS affects the expression kinetics of horizontally acquired genes.
The PhoP/PhoQ regulatory system is a master regulator of virulence and Mg2+ homeostasis in S. enterica serovar Typhimurium and other enteric species (10). The sensor PhoQ responds to low Mg2+ (11), acidic pH (12), and antimicrobial peptides (13) by promoting the phosphorylated state of the regulatory protein PhoP (PhoP-P). In vivo, PhoP-P is the PhoP form that binds to target promoter sequences (14) and recruits RNAP (15) to initiate gene transcription.
The phoP and phoQ genes are ancestral to enteric bacteria (16, 17). In contrast, the majority of PhoP-activated genes in Salmonella and Yersinia pestis appear to have been acquired by horizontal gene transfer (17). Interestingly, within Salmonella, the promoter architecture of PhoP-activated ancestral genes differs from the promoter architecture of PhoP-activated horizontally acquired genes (18). (We refer to promoter architecture as the combination of the number, location, and orientation of binding sites for a transcription factor in a given promoter. Assignment of PhoP-activated genes as ancestral versus horizontally acquired was based on their phylogenetic conservation and GC content [18].) Furthermore, the promoter architecture of PhoP-activated ancestral genes is conserved between Salmonella and Yersinia, whereas species-specific promoter architectures drive transcription of horizontally acquired genes in these two species (19).
We now report that the timing of transcription of PhoP-activated genes following a switch to inducing conditions for the PhoQ protein correlates well with the timing of promoter occupancy by the PhoP-P protein in vivo. In contrast, the mRNA levels of PhoP-dependent genes, especially those acquired horizontally, reflect neither the degree of occupancy of the corresponding promoters by PhoP-P in vivo nor the affinity of the purified PhoP-P protein for the PhoP binding sites in vitro. We demonstrate that most of the horizontally acquired genes are transcribed after ancestral genes despite often reaching higher mRNA levels. Furthermore, we establish that the expression delay exhibited by horizontally acquired genes is due to the need to overcome H-NS-mediated silencing. Our findings provide a singular example of gene ancestry dictating expression behavior among genes controlled by a given transcription factor.
RESULTS
Rationale.
According to the law of mass action, and in the absence of additional factors, one would expect that promoters with high affinity for PhoP-P would be bound by PhoP-P at higher levels, and earlier, than promoters with low affinity for PhoP-P. Once PhoP-P is bound, it recruits RNAP to initiate transcription. If RNAP recruitment does not differ substantially between the promoters, one would then anticipate that genes driven by promoters with high affinity for PhoP-P would also be transcribed to higher levels and at earlier times than those driven by promoters with low-affinity PhoP-P sites. To test this particular hypothesis and thus to determine how the transcriptional activator PhoP differentially controls its target genes, we investigated both the mRNA levels of 20 genes directly activated by the PhoP protein and the occupancy of the corresponding promoters by the PhoP-P protein at different times after Salmonella experienced inducing conditions for the PhoQ protein. The analyzed genes differ in both the function of the encoded products and their ancestry, which was assigned based on their conservation among enteric bacteria harboring the PhoP/PhoQ system and also on their GC content (18).
PhoP-activated genes are transcribed at different times and to different levels.
We examined the mRNA levels of 20 genes directly activated by the PhoP protein at six times after shifting bacteria from medium containing repressing (10 mM) to activating (50 µM) Mg2+ concentrations for PhoP’s cognate sensor PhoQ (11, 20), as well as before the shift to inducing conditions. This approach enabled us to examine how mRNA levels change over time in a set of coregulated genes that differ in their ancestry and physiological functions of the encoded gene products.
The mRNAs for all investigated transcripts increased, reached a peak, and then decreased to steady-state levels that were ~30% of the maximum (Fig. 1A), which we refer to as “surge.” This behavior reflects the dynamic changes in the amount of phosphorylated PhoP protein (20) that results from both positive feedback of PhoP-P on the phoPQ promoter (20, 21) and intrinsic negative feedback whereby the sensor PhoQ dephosphorylates PhoP-P sometime after the onset of inducing conditions (20, 22).
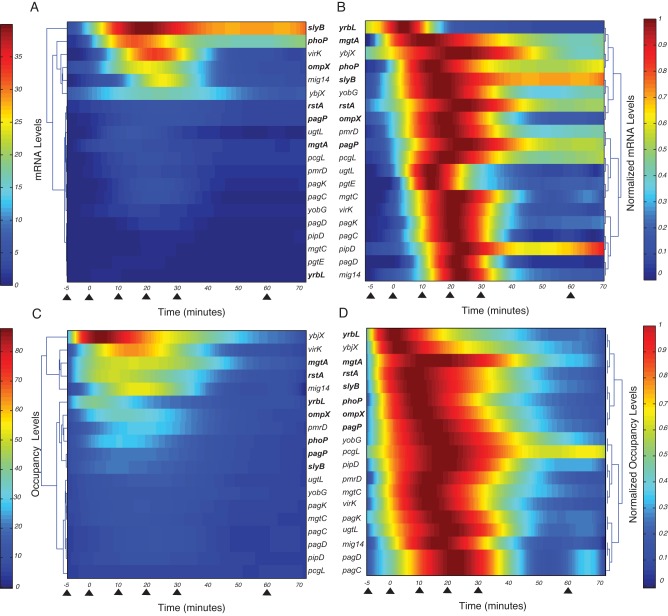
FIG 1 .
mRNA levels of PhoP-activated genes and occupancy by PhoP-P of the corresponding promoters. Ancestral genes are indicated in boldface. (A) Clustering of the mRNA levels for PhoP-dependent genes harvested at 0, 5, 10, 20, 30, 60, and 90 min (triangles) after Salmonella (EG13918) was shifted from medium containing repressing (10 mM) to activating (50 µM) Mg2+ concentrations (triangles; only the first 70 min are shown). The data were smoothed by polynomial interpolation and clustered (see Materials and Methods). The range of mRNA levels is illustrated by the color bar. (B) Clustering of the mRNA levels obtained in panel A normalized to the maximum values for each gene. Grouping was performed by emphasizing the first 40 min to uncover clusters based on the mRNAs onset times (51). (C) Clustering of the in vivo promoter occupancy by the PhoP-P protein determined by ChIP assays using Salmonella (EG13918) grown in 10 mM Mg2+ and then switched to 50 µM Mg2+ and harvested at 0, 5, 10, 20, 30, 60, and 90 min (triangles, only the first 70 min are shown). Bacterial growth, data preprocessing, and clustering were performed as described for panel A. (D) Clustering of the normalized promoter occupancy by the PhoP protein corresponding to the data presented in panel C.
PhoP-activated transcripts during the surge differed both in the levels (i.e., the degree by which individual mRNAs were induced relative to the constitutively expressed PhoP-independent rrs mRNA used as the control) (Fig. 1A; see also Table S1 in the supplemental material) and in the time required for an mRNA to reach 50% of its maximum value (Fig. 1B; see also Table S1) (23, 24). Notably, transcription timing is not well correlated with mRNA levels (see Fig. S1A; F-statistic P value of >0.1). For example, yrbL was the first gene to be transcribed despite being expressed at the lowest levels from all 20 targets. By contrast, mig-14 was the last gene to be transcribed even though its mRNA level was the fifth highest of the investigated targets (Fig. 1A and B). These results indicate that for genes coregulated by the PhoP-P protein, the time at which the corresponding mRNAs are produced is not necessarily correlated with the levels that are achieved.
PhoP-activated promoters are bound by PhoP-P at different times and to different levels.
To explore whether the expression behavior described above reflected binding of the PhoP-P protein to its target promoters in vivo, we carried out chromatin immunoprecipitation (ChIP) and real-time PCR measurement of the recovered DNAs at different time points after organisms experienced the same induction conditions used for the transcription experiments discussed above. PhoP-P bound to all investigated promoters, indicative that PhoP-P plays a direct role in their control. In agreement with the transcription data (Fig. 1A and B), the PhoP-P protein occupied PhoP-activated promoters only transiently (Fig. 1C and D). As with expression timing, promoter occupancy timing was not well correlated with promoter occupancy levels (see Fig. S1B; F-statistics P value of >0.1).
A comparison of the binding (Fig. 1C and D; see also Table S1) and transcription (Fig. 1A and B) data sets demonstrated that promoter occupancy timing does correlate well with the timing of appearance of the corresponding mRNAs (see Fig. S1C; F-statistics P value of <1E−04). By contrast, promoter occupancy levels are only partially correlated with the mRNA levels (see Fig. S1D; F-statistics P value of >0.1). The lack of correlation for a few PhoP-activated genes appears to reflect the involvement of additional factors. For example, the regulatory protein PmrA represses transcription of the yrbL and pmrD genes (25, 26), the latter specifying a product that posttranslationally activates the PmrA protein (27). The mgtA transcript includes a leader region that controls transcription elongation into the coding region in response to the cytoplasmic levels of Mg2+ (28) and proline-charged tRNAPro (29). The rstA promoter harbors both activation and repression sites for the PhoP-P protein (data not shown). When these additional factors were accounted for, there was excellent correlation between promoter occupancy and mRNA levels in the pmrD, yrbL, rstA, and mgtA genes. Thus, these corrections can account for the differences observed in all examined targets (F-statistics P value of <7.53E−5).
Promoters harboring a single PhoP box are bound by PhoP-P at early times and to high levels.
To explore the relationship between promoter occupancy by PhoP-P in vivo and the affinity of the purified PhoP-P for the PhoP boxes of the corresponding promoters in vitro, we performed electrophoretic mobility shift assays (EMSAs) on 34 synthetic oligonucleotides, each harboring a single PhoP box originating from the 20 investigated promoters (see Fig. S2 and Table S2). (The PhoP-P protein recognizes a hexanucleotide direct repeat separated by five nucleotides termed the PhoP box [30]. Seven of the 20 promoters harbor a single PhoP box whereas the remaining 13 promoters contain at least two PhoP boxes [18].)
The PhoP-P protein displayed the highest affinity for the PhoP boxes of promoters with a single PhoP box (see Table S2). These promoters were occupied early and to high levels by the PhoP-P protein in vivo (Fig. 1C and D), resulting in early expression for all seven of these genes (Fig. 1B). The affinity of PhoP-P for the PhoP box in the early genes appears to control transcription timing because replacing the PhoP box from the early phoP promoter (driving expression of a promoterless gfp gene) by 17 nucleotides corresponding to a PhoP box from the late pagK promoter, which is bound less avidly by the PhoP-P protein in vitro (see Fig. S2), delayed its expression in vivo (see Fig. S3).
Promoters harboring two PhoP boxes are bound by PhoP-P at late times and/or to low levels.
The PhoP-P protein bound with high affinity to some of the promoters in this group in vitro (see Fig. S4A and Table S2). For example, the virK promoter harbors two PhoP boxes, both of which are required for PhoP-dependent gene transcription (18). One of the virK PhoP boxes has higher affinity for PhoP-P than the single PhoP box in the early phoP promoter (see Fig. S4A and Table S2). Correspondingly, the in vivo occupancy level of the virK promoter by PhoP-P was the second highest of the 20 investigated promoters (Fig. 1C), as expected for a promoter with high-affinity PhoP boxes (see Fig. S4B and Table S2). Anomalously, the PhoP-P protein occupied the virK promoter later than the phoP promoter in vivo (Fig. 1D).
Similarly, PhoP-P has a higher affinity for the PhoP boxes in the virK promoter than for those in the pcgL promoter in vitro (see Fig. S4A and B and Table S2). If binding of PhoP-P in vivo reflected the affinities of the purified PhoP-P for its binding sites in vitro, then PhoP-P binding to the virK promoter should reach higher levels than and precede binding to the pcgL promoter. Even though PhoP-P binding to the virK promoter did reach higher levels than to the pcgL promoter (Fig. 1C), it took place at later times (Fig. 1D). The pcgL promoter has PhoP boxes of intermediate in vitro affinity for PhoP-P (see Fig. S4A and B and Table S2) and displays intermediate binding time in vivo. However, the in vivo occupancy level by the PhoP-P protein was the lowest of the 20 investigated promoters (Fig. 1C).
The anomalously late PhoP-P binding to the virK promoter and the anomalously low binding to the pcgL promoter suggest that there is a factor(s) that delays PhoP-P binding to the virK promoter and/or dampens binding to the pcgL promoter in vivo. This factor(s) might also affect the expression timing and expression level of other promoters harboring two PhoP boxes as all 13 PhoP-activated promoters that harbor two PhoP boxes were bound late and/or to low levels by PhoP-P in vivo (Fig. 1C and D).
The promoter architectures of PhoP-activated genes correlate with PhoP-P binding behavior.
The profiles of promoter occupancy timing by the PhoP-P protein in vivo are correlated with the five promoter architectures driving transcription of PhoP-activated genes (18). Specifically, early promoter occupancy was displayed by promoters activating transcription of ancestral genes (e.g., phoP; see Table S2), whereas late promoter occupancy corresponded to architectures promoting transcription of horizontally acquired genes (e.g., pcgL, virK; see Table S2). By contrast, there was no link between the time a promoter was occupied and the function of the transcribed gene product. For instance, the promoters for genes specifying different Mg2+ transporters (i.e., mgtA and mgtB) were occupied both early and late, and the same was true for promoters controlling expression of proteins that modify the lipopolysaccharide (i.e., pagP and ugtL).
H-NS dampens PhoP-activated gene expression.
Because some PhoP-activated horizontally acquired genes were previously shown to be bound by H-NS (5, 6, 15), we wondered whether H-NS might be responsible for the decreased occupancy of horizontally acquired gene promoters by the PhoP-P protein when Salmonella experiences inducing signals for the PhoQ protein. To carry out these experiments, we compared the mRNA levels produced by isogenic rpoS and rpoS hns mutants at different times after organisms grown in LB broth containing 10 mM Mg2+ were switched to LB broth without added Mg2+ to induce the PhoP/PhoQ system (Fig. 2). It was necessary to carry out these experiments in an rpoS genetic background and LB medium because (i) hns is an essential gene in Salmonella, and the rpoS mutation suppresses lethality (5, 6), and (ii) the rpoS hns mutant failed to grow in defined medium.
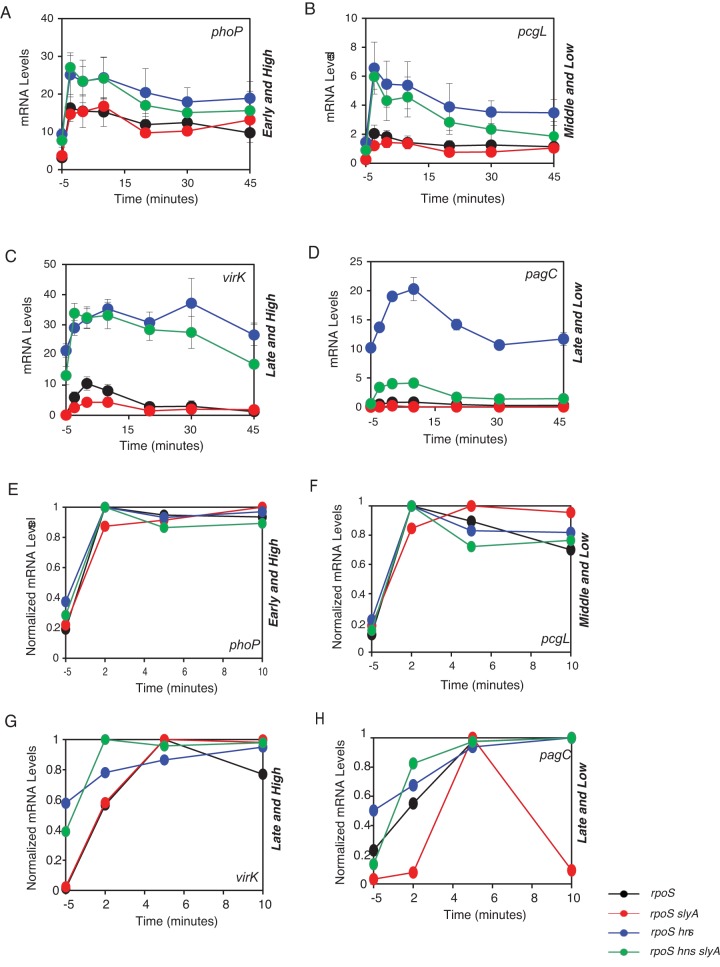
FIG 2 .
H-NS and SlyA influence the levels and timing at which mRNAs for PhoP-activated genes are produced. mRNA levels for PhoP-dependent genes after bacteria were shifted from LB broth containing 10 mM Mg2+ to LB broth. We investigated the H-NS and SlyA-independent phoP gene, the H-NS-dependent and SlyA-independent pcgL gene, and the virK, mig-14, and pagC genes, which are H-NS- and SlyA-dependent. mRNA in the rpoS strain (MJC140), rpoS hns strain (MJC138), rpoS hns slyA strain (MJC139), and rpoS slyA strain (MJC141) displaying four different expression behaviors in terms of timing and levels at which the mRNAs are produced: early and high (phoP) (A), middle and low (pcgL) (B), late and high (virK) (C), and late and low (pagK) (D). (E to H) H-NS and SlyA influence the timing at which mRNAs for PhoP-activated genes are produced. Normalized mRNA levels corresponding to panels A to D displaying differential onset times.
We chose the early gene phoP, the middle gene pcgL, the late gene virK, and the late gene pagC as representatives of the four behaviors that exemplify expression timing and amounts: early and high, early and low, late and high, and late and low, respectively (Fig. 1A and B). We determined that the phoP, pcgL, virK, and pagC genes were transcribed in the rpoS mutant grown in LB broth at times and levels proportional to those displayed by wild-type Salmonella grown in N-minimal medium (Fig. 1A and 2). These results validated the use of the rpoS genetic background and growth conditions.
Inactivation of the hns gene dramatically derepressed middle and late genes (i.e., 3- to 20-fold) without much of an effect (i.e., ~50% increase) on the early gene phoP (Fig. 2A and D). These results are in agreement with previous findings showing that middle and late genes are silenced by H-NS (15, 31, 32). The virK mRNA levels were higher than those corresponding to the phoP and pcgL genes in the rpoS hns mutant (Fig. 2A and C). Given that PhoP-P exhibits higher affinity for the PhoP boxes in the virK promoter than for the PhoP box in the phoP promoter (see Table S2), these results indicate that if H-NS is absent, the mRNA levels of middle and late genes are dictated primarily by the affinity of PhoP-P for the PhoP boxes in the corresponding promoters (see Fig. S2). There was no transcription of PhoP-activated genes in the rpoS hns phoP triple mutant (data not shown), indicating that PhoP-P is essential for transcription of the investigated promoters even in the absence of H-NS.
A requirement for the slyA gene delays expression among H-NS-silenced genes.
Both pcgL and virK are silenced by H-NS (Fig. 2C and D). Why, then, is the pcgL gene transcribed before the virK gene (Fig. 1C) when PhoP-P has higher in vitro affinity for the PhoP boxes in the virK promoter than for those present in the pcgL promoter (see Fig. S4A and Table S2)? We hypothesized that the distinct expression timing might reflect a differential requirement for the DNA binding protein SlyA. This is because (i) SlyA antagonizes H-NS silencing (9, 15, 33) and is required for transcription of several horizontally acquired PhoP-dependent late genes (32, 34), and (ii) PhoP-P is necessary for SlyA activation (34) and/or expression via a feed-forward loop circuit (31), which typically imposes expression delays on target genes (24).
We determined that the mRNA levels corresponding to the early phoP gene (Fig. 2A) and the middle pcgL (Fig. 2B) gene were similar in the rpoS hns slyA triple mutant and the rpoS hns double mutant. This was also the case when we compared the rpoS slyA double mutant to the rpoS single mutant. Thus, pcgL does not require SlyA for expression. This was in contrast to expression of the late virK and pagC genes, which was reduced in the rpoS slyA double mutant relative to that in the rpoS mutant (Fig. 2C and D). These results demonstrated that SlyA is dispensable for transcription of the phoP and pcgL genes but necessary for transcription of the virK and pagC genes.
SlyA’s role in transcription of the pagC gene is not limited to overcoming silencing by H-NS because deletion of the slyA gene compromised pagC transcription even when H-NS was absent (Fig. 2D) whereas it had a minimal effect on the virK mRNA levels (Fig. 2C). In an rpoS hns slyA genetic background, the expression behavior of the virK gene resembles that of the middle pcgL (Fig. 2B) and early phoP (Fig. 2A) genes during the first 20 min postinduction.
Remarkably, the levels of the virK transcript peaked at earlier times in an rpoS hns slyA triple mutant than in the rpoS hns double mutant (Fig. 2G and H). This effect appears to be specific to virK, because the peak times for the early phoP gene and the middle pcgL gene were similar in the two strains (Fig. 2E and F). These results imply that, when H-NS is absent, SlyA interferes with PhoP-dependent activation of virK and potentially of other late genes.
DISCUSSION
We have now established that the expression kinetics of PhoP-activated genes displayed during the first 90 min postinduction is unusual in that it is dictated by gene ancestry: ancestral genes are expressed before horizontally acquired genes (Fig. 1B and D, Fig. 2E to H, and Fig. 3; see also Fig. S1A and C in the supplemental material). Among horizontally acquired genes, those requiring the PhoP-activated SlyA protein for expression (e.g., virK, mig14, pagC) are transcribed after SlyA-independent genes (e.g., pcgL) (Fig. 1B and D, Fig. 2E to H; see also Fig. S1A and C). The observed behavior does not reflect the affinity of PhoP-P for its target sequences determined in vitro (see Fig. S2), but it is correlated with the distinct architectures of the promoters driving transcription of ancestral versus horizontally acquired genes (18), some of the latter requiring SlyA to antagonize H-NS silencing. Moreover, we determined that the time at which a PhoP-activated gene is transcribed does not necessarily correspond to the mRNA levels that are produced (i.e., a late gene may be transcribed at higher levels than an early gene). This behavior is in contrast to that described for other regulons where early genes are transcribed to higher mRNA levels than those transcribed at later times (35) (Fig. 4). We showed that the delay is caused by the need to overcome H-NS-mediated silencing of horizontally acquired genes (Fig. 2E to H).
FIG 3 .
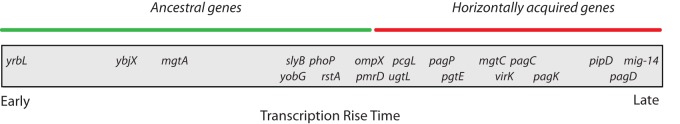
Schematic showing the relationship between gene ancestry and transcription rise time in PhoP-activated genes. The bar represents time in minutes. Relationship between ancestral (green line) and horizontally acquired (red line) genes is indicated.
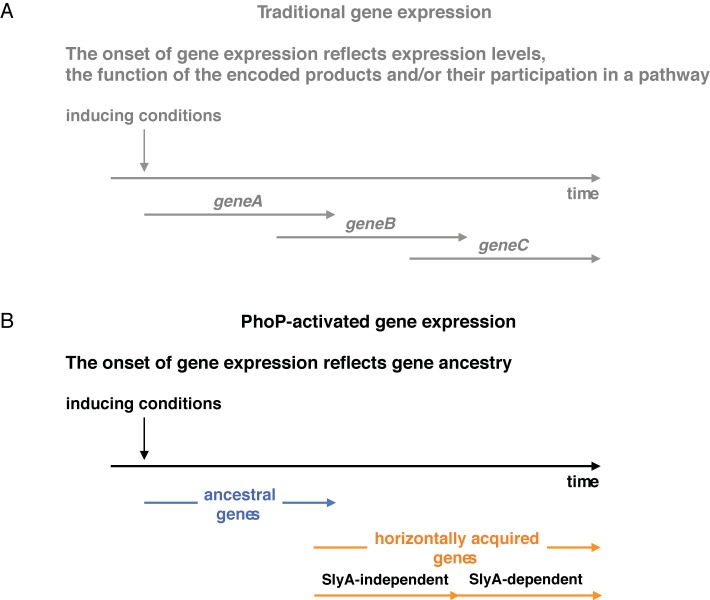
FIG 4 .
Gene ancestry determines the onset of expression of PhoP-activated genes. (A) The traditional view of gene expression timing. The onset of expression of genes controlled by a given regulatory protein reflects the levels at which the mRNA is produced (i.e., early genes are expressed at higher levels than late genes), the function of the encoded products, and/or their participation in a biochemical or morphological pathway. (B) PhoP-activated gene expression. The onset of expression of PhoP-activated genes reflects gene ancestry. Horizontally acquired genes are transcribed after ancestral genes due to the need to overcome H-NS-mediated silencing in the former. Among horizontally acquired genes, those that are dependent on the PhoP-activated SlyA protein are transcribed after those that are SlyA independent.
It is generally assumed that the affinity of a transcriptional activator for its target sequence in a promoter directs the expression levels and expression timing (36). Differential affinity for genes coregulated by a transcription factor can drive distinct developmental pathways. For example, expression of the genes necessary for biofilm formation in Bacillus subtilis requires lower levels of the master regulator Spo0A than expression of those responsible for sporulation (37). This behavior reflects the affinity of the active Spo0A protein for its binding sites in Spo0A-activated promoters. This appears to also be the case for PhoP-activated ancestral genes because replacing a high-affinity PhoP box with a low-affinity PhoP box in an ancestral gene promoter delayed expression (see Fig. S3).
By measuring mRNA levels and promoter occupancy at multiple times after induction (Fig. 1) and determining the affinity of PhoP-P for the individual PhoP boxes in vitro (see Fig. S2 and Table S2), we were able to evaluate these features independently. Our analysis revealed that the kinetics of promoter occupancy and transcription of PhoP-activated horizontally acquired genes observed in vivo cannot be explained by the affinity of PhoP-P for its binding sites. This is because the promoters driving transcription of the horizontally acquired late-expressing virK and mig-14 genes have high-affinity sites for PhoP-P, which are higher than those present in the promoters of genes that are expressed earlier (determined in vitro and inferred from in vivo binding; see Fig. S2 and Table S2). Thus, we considered several possibilities to account for this unusual behavior.
First, PhoP-dependent transcription of horizontally acquired genes may require an additional factor to operate together with the PhoP-P protein. For instance, inducible gene expression by a given transcription factor can be influenced by binding of other transcription factors to the same promoter, which may act as activators in one context and as repressors in a different context (38), activate transcription from promoters recognized by different forms of RNAP (e.g., the GalR [39] and the φ29 [40] proteins), and/or contact distinct subunits of RNAP (e.g., the λcI protein [41]). However, the timing of PhoP-P promoter occupancy correlates well with the timing of mRNA production (F-statistic P value of <1E−04), suggesting that PhoP-P binding per se directly leads to RNAP recruitment and transcription.
Second, the PhoP-P protein bound to all investigated promoters in vivo and in vitro (Fig. 1D; see also Fig. S2), thereby ruling out that the expression delay manifested by PhoP-activated horizontally acquired genes results from indirect regulation. Thus, PhoP is not operating through a classical cascade where delays result from sequential events in regulatory or metabolic pathways (23, 24). Such regulatory cascades are often observed in systems where the products of a set of coregulated genes function sequentially, such as the synthesis of proteins that participate in multistage processes, including morphogenic (e.g., flagellar assembly) and metabolic (e.g., nutrient biosynthetic) pathways (23, 42). By contrast, genes regulated by a transcription factor that do not function in the context of such a temporal program would have no reason, a priori, to exhibit an elaborate temporal expression pattern. Nonetheless, PhoP-activated targets, as well as genes directly regulated by Msn2 in Saccharomyces cerevisiae (43), do display disparate expression timing.
And third, the expression kinetics of PhoP-activated genes reflects the need to overcome silencing of horizontally acquired genes by nucleoid-associated proteins. The effect that H-NS exerts on bacterial promoters (3, 8) is reminiscent of the consequences resulting from packaging of eukaryotic DNA by histones: it typically hinders gene transcription by blocking promoter accessibility by RNAP or transcription factors (TFs) (44, 45). Cells can overcome the silencing effects of nucleoid-associated proteins with specialized factors that modify histones in eukaryotes (44, 46, 47) and that antagonize histone-like proteins in bacteria (33), which enables promoter access by the transcriptional machinery. This process imposes an expression delay in eukaryotic cells (24, 44, 47). This appears to be the case also in bacteria as PhoP-activated genes that are silenced by H-NS are expressed after those that are not silenced by H-NS (Fig. 2). In agreement with this notion, expression of late genes is sped up in the absence of H-NS (Fig. 2E to H). Those late genes that require the PhoP-activated SlyA protein for transcription display a further delay in gene expression (Fig. 2).
We determined that some of the PhoP-activated late genes, such as virK and mig-14, are expressed at higher levels than early genes (Fig. 1A and B; see also Fig. S1). This result indicates that once promoters are cleared of nucleoid-associated proteins, the mRNA levels are dictated by the affinity of RNAP and/or transcription factors for the individual promoter sequences and/or by the arrangement of these sequences (24, 45). The ability to regulate the expression levels independently of the expression timing would not be possible if the mechanism underlying the delay was based solely on the affinity of PhoP-P for its target sequences.
The complex expression program displayed by the Salmonella PhoP-P protein requires promoters with different properties as well as tight control over the levels of PhoP-P. The transcriptional behavior of PhoP-activated genes (Fig. 1) can now be linked to the five identified promoter architectures present in PhoP-activated promoters (18). Salmonella controls PhoP-P levels both by PhoP-P exerting positive feedback on the phoP promoter (20) and by PhoQ exercising negative feedback on the levels of PhoP-P (22). Finally, the participation of nucleoid-associated proteins in bacterial gene silencing highlights the difficulty in making accurate predictions on gene expression activities exclusively from genome sequence analysis.
MATERIALS AND METHODS
Bacterial strains, plasmids, primers, and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table S3 in the supplemental material, and the primers used in this study are listed in Tables S4 to S6. All S. enterica serovar Typhimurium strains used in this study were derived from the wild-type strain 14028s. Bacteria were grown at 37°C in Luria-Bertani (LB) broth or in N-minimal medium, pH 7.7, supplemented with 0.1% Casamino Acids, 38 mM glycerol, and 50 µM or 10 mM MgCl2 (14, 48). When necessary, antibiotics were added at the following final concentrations: ampicillin, 50 µg/ml; kanamycin, 25 µg/ml; and tetracycline, 10 µg/ml. Escherichia coli DH5α was used as a host of plasmid for the preparation DNA.
Construction of plasmids.
Plasmid pMS-phoP4, encoding a shortened derivative of the phoP promoter region including the PhoP box and the RNAP binding site fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8142 and 8143 between BamHI and XhoI sites of plasmid pMS201 (49).
Plasmid pMS-phoP4-pagK, encoding the phoP4 promoter region where the PhoP box was replaced by a PhoP box from the pagK promoter and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 9171 and 9173 between BamHI and XhoI sites of plasmid pMS201 (49).
Plasmid pGRG36-phoP4 was constructed using pMS-phoP4 as the template, where primers 9403 and 9637 were utilized to amplify the gfp gene. The PCR products were digested with XhoI/NotI and ligated with pGRG36(XhoI/NotI) (50). This plasmid was transformed into EG13918 for specific insertion at the chromosomal att::Tn7 site.
Plasmid pGRG36-phoP4-pagK was constructed using pMS-phoP4-pagK as the template, where primers 9403 and 9637 were utilized to amplify the gfp gene. The PCR products were digested with XhoI/NotI and ligated with pGRG36(XhoI/NotI) (50). This plasmid was transformed into EG13918 for specific insertion at the chromosomal att::Tn7 site.
In vivo expression assays.
Time course analysis of mRNA expression in N-minimal medium was performed as follows (20). Bacterial cells were grown in N-minimal medium (pH 7.7) supplemented with 10 mM MgCl2 to mid-exponential phase. Cells were concentrated using a small volume of N-minimal medium with 10 mM MgCl2. To activate transcription of the PhoP-regulated genes, the cell suspension was diluted to prewarmed medium, and the concentration of MgCl2 in the medium was adjusted to 50 µM. Aliquots of cells were removed during growth at the designated times and mixed with RNAprotect bacterial reagent (Qiagen) for stabilization of RNA, and total RNA was isolated using the RNeasy kit (Qiagen). After DNase treatment of the total RNA solution, cDNA was synthesized using Omnitranscript reverse transcription reagents (Qiagen) and random hexamers. Quantification of cDNA was carried out using SYBR green PCR master mix (Applied Biosystems), and real-time amplification of PCR product was analyzed by using an ABI 7000 sequence detection system (Applied Biosystems). The relative amount of cDNA was calculated using a standard curve obtained from PCR on serially diluted genomic DNA as the templates. RNA levels of target genes were normalized to those of the 16S rRNA gene. The sequences of the primers used are presented in Table S5B. Data correspond to the averages from at least three independent experiments, which were smoothed (see “Data preprocessing”) for clustering purposes.
Time course analysis of mRNA expression in organisms grown in LB broth was performed as follows. Salmonella overnight cultures grown in LB medium with 10 mM MgCl2 were washed twice with LB and used to inoculate LB medium containing 10 mM MgCl2 with a 1:50 dilution. Cultures were grown to an optical density at 600 nm (OD600) of ≈0.5 in a shaking water bath at 37°C. To activate transcription of the PhoP-regulated genes, cells were collected and washed with LB once and were shifted to prewarmed LB. Aliquots of cells were removed at the designated times, and cDNA synthesis and quantification of transcripts were carried out as described above. Data correspond to the means ± standard deviations (SD) from at least three independent experiments.
Chromatin immunoprecipitation experiments.
Time course chromatin immunoprecipitation assay was performed as follows (20). Bacterial cells grown in N-minimal medium with 10 mM MgCl2 were shifted to the medium containing 50 µM MgCl2 as described above. Aliquots of cells obtained at the designated times were cross-linked using 1% of formaldehyde, and the ChIP assay was performed as described (20). Briefly, DNA was fragmented to an average size of 500 bp by sonication, and the protein-DNA complex was immunoprecipitated using polyclonal antihemagglutinin antibody (Sigma) and protein G Sepharose (Amersham). Both immunoprecipitated DNA (IP DNA) and preimmunoprecipitated “input DNA” were purified using a Qiagen PCR purification column and were quantified using real-time PCR. To obtain values for PhoP binding to a target promoter, the signal ratio of IP to input was normalized to that of the endogenous control, the rpoD promoter, which is not PhoP regulated (20). All promoter-specific primers are listed in Table S5A. The raw data were smoothed (see “Data preprocessing”).
Electrophoretic mobility shift assay.
The DNA fragments used as probes for this assay were generated as follows. (i) PCR fragments were generated by using plasmid pMS201 as the template and primer 9252 (pMS201-GFP-5′) (5′ CCAGTTTACTTTGCAGGGCTTC 3′) with each of the following primers: 9320, 9322, 9324, 9326, 9328, 9330, 9332, 9334, 9336, 9338, 9340, 9342, 9344, 9346, 9348, 9350, 9352, 9354, 9356, 9358, 9360, 9362, 9364, 9366, 9368, 9370, 9372, 9374, 9376, 9378. The DNA fragments were then gel purified with QIAquick columns (Qiagen). (ii) Sets of PCR fragments were generated by using plasmid pMS201 as the template and primer 7817 (pMS201-GFP-3′) (5′ GCCCATTAACATCACCATC 3′) with each of following primers: 9319, 9321, 9323, 9325, 9327, 9329, 9331, 9333, 9335, 9337, 9339, 9341, 9343, 9345, 9347, 9349, 9351, 9353, 9355, 9357, 9359, 9361, 9363, 9365, 9367, 9369, 9371, 9373, 9375, 9377. The DNA fragments were then gel purified with QIAquick columns (Qiagen). (iii) The PCR fragments from steps i and ii were then mixed and were used as the templates for generating PCR fragments using primers 9252 (5′-CCAGTTTACTTTGCAGGGCTTC-3) and 7817 (5′ GCCCATTAACATCACCATC 3′). The DNA fragments were then gel purified with QIAquick columns (Qiagen). (iv) A total of 150 ng of DNA from step iii was then labeled with 2 units of T4 polynucleotide kinase and 10 pmol of [γ-32P]ATP, and then unincorporated 32P was removed using ProbeQuant G-50 microcolumns (GE Healthcare). Phosphorylation of PhoP-His6 with acetyl phosphate: 1.2 nmol of PhoP-His6 protein was incubated at room temperature for 2.5 h in 50 µl of a buffer consisting of 10 µl of 5× phosphorylation buffer (250 mM Tris [pH 7.5], 250 mM KCl, 100 mM MgCl2) and 5 µl of 100 mM acetyl phosphate (Sigma) and nuclease-free water. Phosphorylated protein was then cleaned up with preequilibrated (two times each with 500 µl of 50 ml Tris [pH 8.0]) Micro Bio-Spin 6 (Bio-Rad). The gel mobility shift assays were carried out as described in reference 15 with freshly made phosphorylated proteins, and samples were then electrophoresed on 4% to 20% Tris-borate-EDTA (TBE) gels (Life Technologies) on 0.38× TBE running buffer at V = 100 for 3 h.
Estimation of PhoP-P binding to multiple PhoP boxes in a promoter calculated from apparent affinities.
Let R be the regulator and P the promoter with two R-binding sites, and assume that the binding to the two sites is independent, where ki = dissociation constant for R binding to site i.
By definition, at equilibrium (omitting brackets):
| (1) |
and
| (2) |
where Rf = concentration of free R. From equation 1:
| (3) |
and
| (4) |
From equation 2:
| (5) |
| (6) |
Let the concentration of the promoter be P = P00 + P01 + P10 + P11. Substituting in equations 3, 5, and 6:
Simplifying, k1k2P = P00(k1k2 + Rfk1 + Rfk2 + Rf2) = P00(k1 + Rf)(k2 + Rf) or
Further,
where
Thus, the relative concentrations of the 4 states (or the relative promoter occupancies) are as follows: P00:P01:P10:P11 = k1k2:k1Rf:k2Rf:Rf2. Rf may be approximated by R when there are many more regulator molecules than promoter molecules, so that the amount of R that is bound to the promoters (P01 + P10 + 2P11) can be ignored. For example, Rf ~ R = 0.9 µM at the peak of the PhoP-P surge (assuming 10% of PhoP is phosphorylated) is much greater than the concentration of the promoters. Occupancy describes the fractional occupancies of the individual binding sites with respect to P.
Data preprocessing.
The raw data corresponding to the mRNA and chromatin immunoprecipitation signals were smoothed by a shape-preserving interpolant-fitting algorithm (Piecewise Cubic Hermite Interpolating Polynomial; MatLab 7.1) that finds values of an underlying interpolating function at intermediate points not described in the experimental assays. We applied a polynomial fit (sixth order) on each expression signal. This smoothing procedure captured the dynamic well, while removing the noise inherent in the differentiation of noisy signals (35).
Calculation of clusters and cluster rankings.
We applied a hierarchical agglomerative clustering (Statistical Toolbox; MatLab 2007b) with a complete linkage method and correlation similarity measurement to group time courses. The function that controls the vertical order in which a row is plotted (Spotfire Decision Site 9.1.2) in a hierarchical clustering is defined as follows. Given two subclusters within a cluster (there are always exactly two subclusters), both subclusters are weighted and the subcluster with the highest weight is placed above the other subcluster. This function is systematically applied until a single cluster containing all rows is obtained. To calculate the weight w3 of a new cluster C3 formed from two subclusters C1 and C2 with a weight of w1 and w2, and each containing n1 and n2 rows, the following expression is used:
The weight of a subcluster with a single row is calculated as the average value of its columns.
SUPPLEMENTAL MATERIAL
Correlations between the time and level of PhoP-activated mRNAs and also with the occupancy of the corresponding promoters by PhoP-P. (A to D) Scatter plot between the rank orders (see “Calculation of clusters and cluster rankings” in the text) of genes derived from clustering the mRNA levels and time at which they are produced and the occupancy of the corresponding promoters by PhoP-P (Fig. 1). The dashed green lines describe the linear correlation among genes. Green and red boxes designate good and bad correlations, respectively. (A) Linear correlation between the time and the levels at which PhoP-activated mRNAs are produced. (B) Linear correlation between the levels and the time of promoter binding by the PhoP-P protein. (C) Linear correlation between promoter binding time by the PhoP-P protein and transcription time of the corresponding genes. (D) Linear correlation between promoter binding levels by PhoP-P and mRNA levels for PhoP-activated genes. Download
In vitro binding of PhoP-P to oligonucleotides containing PhoP boxes from PhoP-activated promoters driving transcription of the genes analyzed in vivo. EMSAs were carried out as described in Materials and Methods with purified PhoP-His6-P protein and DNA fragments harboring the 19 nucleotides corresponding to the PhoP box flanked by the same sequences for all PhoP boxes. Sequences corresponding to each PhoP box and the affinity constants are described in Table S2 (zwiI corresponds to a control sequence with no PhoP box). The x axis constitutes the amount of PhoP-P (pmol). The affinity at 50% of occupancy (Kd) was derived from polynomial fit (sixth order, see “Data preprocessing” in the text). Binding sites corresponding to promoters of ancestral genes are underlined in blue. Binding sites corresponding to promoters of horizontally acquired genes that are silenced by H-NS are underlined in red if their expression is SlyA-independent and not underlined if their expression is SlyA-dependent. The control promoter is underlined in gray. Binding sites corresponding to promoters of horizontally acquired genes not regulated by H-NS or SlyA are underlined in green. Download
Transcription time of an early PhoP-activated gene is determined by the affinity of the PhoP-P protein for its target promoter. Normalized mRNA levels of the phoP gene (blue line) or gfp gene (red line) in strain EG19625 (harboring the phoP4 derivative of the early phoP promoter driving transcription of a promoterless gfp gene located at att::Tn7). Normalized mRNA levels of the phoP gene (green line) or gfp gene (cyan line) in strain EG19627 (harboring a phoP4 promoter derivative where the PhoP box was replaced by a PhoP box from the late pagK promoter). In strain EG19625, the mRNA timing for gfp is the same as that of the phoP gene originating from its normal chromosomal location. By contrast, in strain EG19627, the mRNA timing for gfp is delayed with respect to the timing of the phoP gene transcribed from its normal chromosomal location. Download
Correlations between the rank orders of promoters derived from clustering the time (Fig. 1D) and levels (Fig. 1C) at which they are occupied in vivo by PhoP-P and their corresponding PhoP-P binding derived from in vitro measurements (see “Calculation of clusters and cluster rankings” in the text). (A) Linear correlation (central dashed line) between PhoP-P binding to the PhoP box exhibiting the maximum affinity (i.e., minimum Kd) in a promoter (x axis; see Table S2) and its corresponding rank order of in vivo occupancy (y axis; Fig. 1D; 1:early 20:late). Square colors reflect the time frame measured in minutes where distinct promoters achieve 50% of PhoP-P binding. Promoters with low correlation diverge from the central correlation line (e.g., the virK promoter). (B) Linear correlation (central dashed line) between PhoP-P binding to all PhoP boxes (total binding) in a promoter (x axis; Table S2) and its corresponding rank level of in vivo occupancy (y axis; Fig. 1C; 1:low 20:high). Square colors reflect the range of peak levels achieved by PhoP-P binding to the different promoters. Promoters with low correlation diverge from the central correlation line (e.g., the pcgL promoter). Download
Functional and expression properties of 20 PhoP-activated genes. “Rise time” corresponds to the time required to achieve 50% of the maximum signal (Fig. 1); “label” indicates a clustering category (Fig. 1), and “A/LA” indicates gene ancestry: ancestral/horizontally acquired.
Data set corresponding to the 20 PhoP-activated promoters analyzed in this paper. “Code” indicates the corresponding panel in Fig. S2; “H” and “H/S” stand for H-NS- and H-NS- and SlyA-dependent, respectively (data not shown) (3–5, 8, 9, 15, 31, 33, 34); “PhoP-P binding” describes the fractional occupancies of the PhoP box(es), where P00, P10, P01, P11 and total correspond to different states of binding: two boxes empty, occupancy of the first box, occupancy of the second box, and occupancy of both boxes (see above); “−” indicates very small values unable to be measured; ancestral genes are indicated in bold.
Bacterial strains used in this study.
Promoter-specific primers used in EMSA.
(A) Promoter-specific primers used in ChIP assay; (B) primers used in transcription assay.
ACKNOWLEDGMENTS
We thank Michael Cromie for the construction of strains and Jennifer Aronson for help with the preparation of the manuscript.
This work was supported, in part, by grant AI49561 from the National Institutes of Health to E.A.G., who is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Citation Zwir I, Yeo W-S, Shin D, Latifi T, Huang H, Groisman EA. 2014. Bacterial nucleoid-associated protein uncouples transcription levels from transcription timing. mBio 5(5):e01485-14. doi:10.1128/mBio.01485-14.
REFERENCES
- 1. Browning DF, Busby SJ. 2004. The regulation of bacterial transcription initiation. Nat. Rev. Microbiol. 2:57–65. 10.1038/nrmicro787 [DOI] [PubMed] [Google Scholar]
- 2. Lee DJ, Minchin SD, Busby SJ. 2012. Activating transcription in bacteria. Annu. Rev. Microbiol. 66:125–152. 10.1146/annurev-micro-092611-150012 [DOI] [PubMed] [Google Scholar]
- 3. Dorman CJ. 2004. H-NS: a universal regulator for a dynamic genome. Nat. Rev. Microbiol. 2:391–400. 10.1038/nrmicro883 [DOI] [PubMed] [Google Scholar]
- 4. Dorman CJ. 2007. H-NS, the genome sentinel. Nat. Rev. Microbiol. 5:157–161. 10.1038/nrmicro1598 [DOI] [PubMed] [Google Scholar]
- 5. Navarre WW, Porwollik S, Wang Y, McClelland M, Rosen H, Libby SJ, Fang FC. 2006. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science 313:236–238. 10.1126/science.1128794 [DOI] [PubMed] [Google Scholar]
- 6. Lucchini S, Rowley G, Goldberg MD, Hurd D, Harrison M, Hinton JC. 2006. H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathog. 2:e81. 10.1371/journal.ppat.0020081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oshima T, Ishikawa S, Kurokawa K, Aiba H, Ogasawara N. 2006. Escherichia coli histone-like protein H-NS preferentially binds to horizontally acquired DNA in association with RNA polymerase. DNA Res. 13:141–153. 10.1093/dnares/dsl009 [DOI] [PubMed] [Google Scholar]
- 8. Dorman CJ, Kane KA. 2009. DNA bridging and antibridging: a role for bacterial nucleoid-associated proteins in regulating the expression of laterally acquired genes. FEMS Microbiol. Rev. 33:587–592. 10.1111/j.1574-6976.2008.00155.x [DOI] [PubMed] [Google Scholar]
- 9. Navarre WW, McClelland M, Libby SJ, Fang FC. 2007. Silencing of xenogeneic DNA by H-NS-facilitation of lateral gene transfer in bacteria by a defense system that recognizes foreign DNA. Genes Dev. 21:1456–1471. 10.1101/gad.1543107 [DOI] [PubMed] [Google Scholar]
- 10. Groisman EA. 2001. The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 183:1835–1842. 10.1128/JB.183.6.1835-1842.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. García Véscovi E, Soncini FC, Groisman EA. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84:165–174. 10.1016/S0092-8674(00)81003-X [DOI] [PubMed] [Google Scholar]
- 12. Prost LR, Daley ME, Le Sage V, Bader MW, Le Moual H, Klevit RE, Miller SI. 2007. Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol. Cell 26:165–174. 10.1016/j.molcel.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 13. Bader MW, Sanowar S, Daley ME, Schneider AR, Cho U, Xu W, Klevit RE, Le Moual H, Miller SI. 2005. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell 122:461–472. 10.1016/j.cell.2005.05.030 [DOI] [PubMed] [Google Scholar]
- 14. Shin D, Groisman EA. 2005. Signal-dependent binding of the response regulators PhoP and PmrA to their target promoters in vivo. J. Biol. Chem. 280:4089–4094. 10.1074/jbc.M412741200 [DOI] [PubMed] [Google Scholar]
- 15. Perez JC, Latifi T, Groisman EA. 2008. Overcoming H-NS-mediated transcriptional silencing of horizontally acquired genes by the PhoP and SlyA proteins in Salmonella enterica. J. Biol. Chem. 283:10773–10783. 10.1074/jbc.M709843200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Groisman EA, Chiao E, Lipps CJ, Heffron F. 1989. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc. Natl. Acad. Sci. U. S. A. 86:7077–7081. 10.1073/pnas.86.18.7077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perez JC, Shin D, Zwir I, Latifi T, Groisman EA. 2009. Evolution of a bacterial regulon controlling virulence and Mg2+ homeostasis. PLoS Genet. 5:e1000428. 10.1371/journal.pgen.1000428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zwir I, Latifi T, Perez JC, Huang H, Groisman EA. 2012. The promoter architectural landscape of the Salmonella PhoP regulon. Mol. Microbiol. 84:463–485. 10.1111/j.1365-2958.2012.08036.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perez JC, Groisman EA. 2009. Transcription factor function and promoter architecture govern the evolution of bacterial regulons. Proc. Natl. Acad. Sci. U. S. A. 106:4319–4324. 10.1073/pnas.0810343106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shin D, Lee EJ, Huang H, Groisman EA. 2006. A positive feedback loop promotes transcription surge that jump-starts Salmonella virulence circuit. Science 314:1607–1609. 10.1126/science.1134930 [DOI] [PubMed] [Google Scholar]
- 21. Soncini FC, Véscovi EG, Groisman EA. 1995. Transcriptional autoregulation of the Salmonella typhimurium phoPQ operon. J. Bacteriol. 177:4364–4371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yeo WS, Zwir I, Huang HV, Shin D, Kato A, Groisman EA. 2012. Intrinsic negative feedback governs activation surge in two-component regulatory systems. Mol. Cell 45:409–421. 10.1016/j.molcel.2011.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chechik G, Oh E, Rando O, Weissman J, Regev A, Koller D. 2008. Activity motifs reveal principles of timing in transcriptional control of the yeast metabolic network. Nat. Biotechnol. 26:1251–1259. 10.1038/nbt.1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yosef N, Regev A. 2011. Impulse control: temporal dynamics in gene transcription. Cell 144:886–896. 10.1016/j.cell.2011.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kato A, Latifi T, Groisman EA. 2003. Closing the loop: the PmrA/PmrB two-component system negatively controls expression of its posttranscriptional activator PmrD. Proc. Natl. Acad. Sci. U. S. A. 100:4706–4711. 10.1073/pnas.0836837100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zwir I, Shin D, Kato A, Nishino K, Latifi T, Solomon F, Hare JM, Huang H, Groisman EA. 2005. Dissecting the PhoP regulatory network of Escherichia coli and Salmonella enterica. Proc. Natl. Acad. Sci. U. S. A. 102:2862–2867. 10.1073/pnas.0408238102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kato A, Groisman EA. 2004. Connecting two-component regulatory systems by a protein that protects a response regulator from dephosphorylation by its cognate sensor. Genes Dev. 18:2302–2313. 10.1101/gad.1230804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cromie MJ, Shi Y, Latifi T, Groisman EA. 2006. An RNA sensor for intracellular Mg(2+). Cell 125:71–84. 10.1016/j.cell.2006.01.043 [DOI] [PubMed] [Google Scholar]
- 29. Park SY, Cromie MJ, Lee EJ, Groisman EA. 2011. A bacterial mRNA leader that employs different mechanisms to sense disparate intracellular signals. Cell 142:737–748. 10.1016/j.cell.2010.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kato A, Tanabe H, Utsumi R. 1999. Molecular characterization of the PhoP-PhoQ two-component system in Escherichia coli K-12: identification of extracellular Mg2+-responsive promoters. J. Bacteriol. 181:5516–5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shi Y, Latifi T, Cromie MJ, Groisman EA. 2004. Transcriptional control of the antimicrobial peptide resistance ugtL gene by the Salmonella PhoP and SlyA regulatory proteins. J. Biol. Chem. 279:38618–38625. 10.1074/jbc.M406149200 [DOI] [PubMed] [Google Scholar]
- 32. Zhao G, Weatherspoon N, Kong W, Curtiss R, III, Shi Y. 2008. A dual-signal regulatory circuit activates transcription of a set of divergent operons in Salmonella typhimurium. Proc. Natl. Acad. Sci. U. S. A. 105:20924–20929. 10.1073/pnas.0807071106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stoebel DM, Free A, Dorman CJ. 2008. Anti-silencing: overcoming H-NS-mediated repression of transcription in Gram-negative enteric bacteria. Microbiology 154:2533–2545. 10.1099/mic.0.2008/020693-0 [DOI] [PubMed] [Google Scholar]
- 34. Navarre WW, Halsey TA, Walthers D, Frye J, McClelland M, Potter JL, Kenney LJ, Gunn JS, Fang FC, Libby SJ. 2005. Co-regulation of Salmonella enterica genes required for virulence and resistance to antimicrobial peptides by SlyA and PhoP/PhoQ. Mol. Microbiol. 56:492–508. 10.1111/j.1365-2958.2005.04553.x [DOI] [PubMed] [Google Scholar]
- 35. Ronen M, Rosenberg R, Shraiman BI, Alon U. 2002. Assigning numbers to the arrows: parameterizing a gene regulation network by using accurate expression kinetics. Proc. Natl. Acad. Sci. U. S. A. 99:10555–10560. 10.1073/pnas.152046799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rajkumar AS, Dénervaud N, Maerkl SJ. 2013. Mapping the fine structure of a eukaryotic promoter input-output function. Nat. Genet. 45:1207–1215. 10.1038/ng.2729 [DOI] [PubMed] [Google Scholar]
- 37. Fujita M, González-Pastor JE, Losick R. 2005. High- and low-threshold genes in the Spo0A regulon of Bacillus subtilis. J. Bacteriol. 187:1357–1368. 10.1128/JB.187.4.1357-1368.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roy S, Semsey S, Liu M, Gussin GN, Adhya S. 2004. GalR represses galP1 by inhibiting the rate-determining open complex formation through RNA polymerase contact: a GalR negative control mutant. J. Mol. Biol. 344:609–618. 10.1016/j.jmb.2004.09.070 [DOI] [PubMed] [Google Scholar]
- 39. Roy S, Garges S, Adhya S. 1998. Activation and repression of transcription by differential contact: two sides of a coin. J. Biol. Chem. 273:14059–14062. 10.1074/jbc.273.23.14059 [DOI] [PubMed] [Google Scholar]
- 40. Monsalve M, Calles B, Mencía M, Salas M, Rojo F. 1997. Transcription activation or repression by phage psi 29 protein p4 depends on the strength of the RNA polymerase-promoter interactions. Mol. Cell 1:99–107. 10.1016/S1097-2765(00)80011-8 [DOI] [PubMed] [Google Scholar]
- 41. Li M, McClure WR, Susskind MM. 1997. Changing the mechanism of transcriptional activation by phage lambda repressor. Proc. Natl. Acad. Sci. U. S. A. 94:3691–3696. 10.1073/pnas.94.8.3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shen-Orr SS, Milo R, Mangan S, Alon U. 2002. Network motifs in the transcriptional regulation network of Escherichia coli. Nat. Genet. 31:64–68. 10.1038/ng881 [DOI] [PubMed] [Google Scholar]
- 43. Hao N, O’Shea EK. 2012. Signal-dependent dynamics of transcription factor translocation controls gene expression. Nat. Struct. Mol. Biol. 19:31–39. 10.1038/nsmb.2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Floer M, Bryant GO, Ptashne M. 2008. HSP90/70 chaperones are required for rapid nucleosome removal upon induction of the GAL genes of yeast. Proc. Natl. Acad. Sci. U. S. A. 105:2975–2980. 10.1073/pnas.0800053105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim HD, O’Shea EK. 2008. A quantitative model of transcription factor-activated gene expression. Nat. Struct. Mol. Biol. 15:1192–1198. 10.1038/nsmb.1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Henikoff S. 2008. Nucleosome destabilization in the epigenetic regulation of gene expression. Nat. Rev. Genet. 9:15–26. 10.1038/nrg2206 [DOI] [PubMed] [Google Scholar]
- 47. Zawadzki KA, Morozov AV, Broach JR. 2009. Chromatin-dependent transcription factor accessibility rather than nucleosome remodeling predominates during global transcriptional restructuring in Saccharomyces cerevisiae. Mol. Biol. Cell 20:3503–3513. 10.1091/mbc.E09-02-0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Snavely MD, Miller CG, Maguire ME. 1991. The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J. Biol. Chem. 266:815–823 [PubMed] [Google Scholar]
- 49. Mangan S, Alon U. 2003. Structure and function of the feed-forward loop network motif. Proc. Natl. Acad. Sci. U. S. A. 100:11980–11985. 10.1073/pnas.2133841100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McKenzie GJ, Craig NL. 2006. Fast, easy and efficient: site-specific insertion of transgenes into enterobacterial chromosomes using Tn7 without need for selection of the insertion event. BMC Microbiol. 6:39. 10.1186/1471-2180-6-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kalir S, McClure J, Pabbaraju K, Southward C, Ronen M, Leibler S, Surette MG, Alon U. 2001. Ordering genes in a flagella pathway by analysis of expression kinetics from living bacteria. Science 292:2080–2083. 10.1126/science.1058758 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlations between the time and level of PhoP-activated mRNAs and also with the occupancy of the corresponding promoters by PhoP-P. (A to D) Scatter plot between the rank orders (see “Calculation of clusters and cluster rankings” in the text) of genes derived from clustering the mRNA levels and time at which they are produced and the occupancy of the corresponding promoters by PhoP-P (Fig. 1). The dashed green lines describe the linear correlation among genes. Green and red boxes designate good and bad correlations, respectively. (A) Linear correlation between the time and the levels at which PhoP-activated mRNAs are produced. (B) Linear correlation between the levels and the time of promoter binding by the PhoP-P protein. (C) Linear correlation between promoter binding time by the PhoP-P protein and transcription time of the corresponding genes. (D) Linear correlation between promoter binding levels by PhoP-P and mRNA levels for PhoP-activated genes. Download
In vitro binding of PhoP-P to oligonucleotides containing PhoP boxes from PhoP-activated promoters driving transcription of the genes analyzed in vivo. EMSAs were carried out as described in Materials and Methods with purified PhoP-His6-P protein and DNA fragments harboring the 19 nucleotides corresponding to the PhoP box flanked by the same sequences for all PhoP boxes. Sequences corresponding to each PhoP box and the affinity constants are described in Table S2 (zwiI corresponds to a control sequence with no PhoP box). The x axis constitutes the amount of PhoP-P (pmol). The affinity at 50% of occupancy (Kd) was derived from polynomial fit (sixth order, see “Data preprocessing” in the text). Binding sites corresponding to promoters of ancestral genes are underlined in blue. Binding sites corresponding to promoters of horizontally acquired genes that are silenced by H-NS are underlined in red if their expression is SlyA-independent and not underlined if their expression is SlyA-dependent. The control promoter is underlined in gray. Binding sites corresponding to promoters of horizontally acquired genes not regulated by H-NS or SlyA are underlined in green. Download
Transcription time of an early PhoP-activated gene is determined by the affinity of the PhoP-P protein for its target promoter. Normalized mRNA levels of the phoP gene (blue line) or gfp gene (red line) in strain EG19625 (harboring the phoP4 derivative of the early phoP promoter driving transcription of a promoterless gfp gene located at att::Tn7). Normalized mRNA levels of the phoP gene (green line) or gfp gene (cyan line) in strain EG19627 (harboring a phoP4 promoter derivative where the PhoP box was replaced by a PhoP box from the late pagK promoter). In strain EG19625, the mRNA timing for gfp is the same as that of the phoP gene originating from its normal chromosomal location. By contrast, in strain EG19627, the mRNA timing for gfp is delayed with respect to the timing of the phoP gene transcribed from its normal chromosomal location. Download
Correlations between the rank orders of promoters derived from clustering the time (Fig. 1D) and levels (Fig. 1C) at which they are occupied in vivo by PhoP-P and their corresponding PhoP-P binding derived from in vitro measurements (see “Calculation of clusters and cluster rankings” in the text). (A) Linear correlation (central dashed line) between PhoP-P binding to the PhoP box exhibiting the maximum affinity (i.e., minimum Kd) in a promoter (x axis; see Table S2) and its corresponding rank order of in vivo occupancy (y axis; Fig. 1D; 1:early 20:late). Square colors reflect the time frame measured in minutes where distinct promoters achieve 50% of PhoP-P binding. Promoters with low correlation diverge from the central correlation line (e.g., the virK promoter). (B) Linear correlation (central dashed line) between PhoP-P binding to all PhoP boxes (total binding) in a promoter (x axis; Table S2) and its corresponding rank level of in vivo occupancy (y axis; Fig. 1C; 1:low 20:high). Square colors reflect the range of peak levels achieved by PhoP-P binding to the different promoters. Promoters with low correlation diverge from the central correlation line (e.g., the pcgL promoter). Download
Functional and expression properties of 20 PhoP-activated genes. “Rise time” corresponds to the time required to achieve 50% of the maximum signal (Fig. 1); “label” indicates a clustering category (Fig. 1), and “A/LA” indicates gene ancestry: ancestral/horizontally acquired.
Data set corresponding to the 20 PhoP-activated promoters analyzed in this paper. “Code” indicates the corresponding panel in Fig. S2; “H” and “H/S” stand for H-NS- and H-NS- and SlyA-dependent, respectively (data not shown) (3–5, 8, 9, 15, 31, 33, 34); “PhoP-P binding” describes the fractional occupancies of the PhoP box(es), where P00, P10, P01, P11 and total correspond to different states of binding: two boxes empty, occupancy of the first box, occupancy of the second box, and occupancy of both boxes (see above); “−” indicates very small values unable to be measured; ancestral genes are indicated in bold.
Bacterial strains used in this study.
Promoter-specific primers used in EMSA.
(A) Promoter-specific primers used in ChIP assay; (B) primers used in transcription assay.