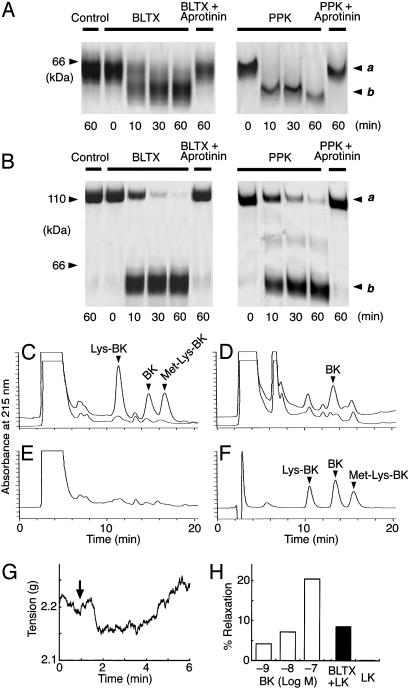
Fig. 4.
Kininogen cleavage activity of BLTX. (A) SDS/PAGE analysis of the degradation of LK by BLTX or PPK at various time intervals and its inhibition by aprotinin. Band a, LK (64 kDa); band b, degraded products (59 kDa). Control, LK incubated without enzymes; + Aprotinin, LK was hydrolyzed by enzymes that have been preincubated with aprotinin (10 μM) for 5 min. (B) SDS/PAGE analysis of the degradation of HK by BLTX or PPK, as described for A. Band a, HK (110 kDa); band b, degraded products (58–63 kDa). (C) HPLC analysis of the peptides released from LK after incubation with (upper trace) and without (lower trace) BLTX. (D) HPLC analysis of the peptides released from HK as described in C. (E) A sample incubated without kininogens. (F) Standard kinins (50 ng each). (G) Transient vasodilation in bovine coronary artery produced by LK treated with BLTX. The artery was precontracted with U46619 (500 nM). Arrow indicates the time of LK injection. (H) Effect of BK-induced and the LK degradation products-induced relaxation on bovine coronary arteries. Results are expressed as percent relaxation of the U46619-treated rings, with 100% relaxation representing the basal tension.